
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Biotech Stock Roundup: Celgene Submits MS Drug To FDA, INSY & CBAY Crash

The biotech sector was back in focus this week with quite a few regulatory updates. While Celgene’s (NASDAQ:CELG) multiple sclerosis (MS) drug was submitted for approval, Vertex expanded its collaboration with CRISPR. Meanwhile, shares of both Insys (NASDAQ:INSY) and CymaBay Therapeutics (NASDAQ:CBAY) plunged on disappointing news.
Recap of the Week’s Top Stories:
Celgene's MS Drug Accepted for Review in US/EU: Celgene announced that the FDA has accepted for review the new drug application (NDA) for ozanimod for the treatment of people with relapsing forms of multiple sclerosis (RMS) in the United States. The European Medicines Agency (EMA) also accepted for review the Marketing Authorization Application for ozanimod for the treatment of adults with relapsing-remitting multiple sclerosis in the European Union.
The FDA has set an action date of Mar 25, 2020. A regulatory decision from the EMA is expected in the first half of 2020. The FDA and EMA applications are based primarily on ozanimod data from the SUNBEAM and RADIANCE Part B phase III studies. In February 2018, the company received a Refusal to File letter from the FDA regarding its NDA for ozanimod for the treatment of people with RMS. Upon its preliminary review, the FDA determined that the nonclinical and clinical pharmacology sections in the NDA were insufficient to permit a complete review. The company then submitted a NDA again in March 2019 to the FDA based on data from the SUNBEAM and RADIANCE studies.
Celgene currently carries a Zacks Rank #2 (Buy). You can see the complete list of today's Zacks #1 Rank (Strong Buy) stocks here.
Insys Crashes on Bankruptcy News: Insys Therapeutics plummeted 51.4% after it filed for bankruptcy protection under Chapter 11 of the U.S. Bankruptcy Code in the U.S. Bankruptcy Court in the District of Delaware. The protection will enable the company to facilitate the sale of substantially all its assets and address its legacy legal liabilities. Insys has been embroiled in various litigations in recent times amid allegations of fueling the ongoing opioid endemic in the United States. The company has also been accused of bribing doctors to prescribe its lead drug, Subsys, to patients. This month, Insys signed a settlement with the United States, formalizing its previously announced settlement relating to certain civil statutory claims. As part of this agreement, the company agreed to pay $195 million.
Biogen Acquires Nightstar: Biogen (NASDAQ:BIIB) announced that it has acquired clinical-stage gene therapy company Nightstar Therapeutics for $800 million. The acquisition will add NST’s lead asset, NSR-REP1, to the company’s pipeline. The candidate is being evaluated for the treatment of choroideremia (CHM), a rare, degenerative, X-linked inherited retinal disorder, which leads to blindness and has no approved treatments. NSR-RPGR is NST’s second clinical program for the treatment of X-linked retinitis pigmentosa (XLRP), which is also a rare inherited retinal disease, primarily affecting males with no approved treatments. Biogen now has added two mid- to late-stage clinical assets, as well as preclinical programs, in ophthalmology.
Vertex Expands Collaboration With CRISPR: Vertex Pharmaceuticals (NASDAQ:VRTX) announced that it is expanding its collaboration with CRISPR Therapeutics (NASDAQ:CRSP) for an exclusive licensing agreement to discover and develop gene editing therapies targeting Duchenne muscular dystrophy (“DMD”) and Myotonic dystrophy type 1 (DM1).
Per the expanded collaboration terms, Vertex will pay CRISPR $1 billion, including $175 million in upfront payment and potential research, development, regulatory, and commercial milestone payments for the DMD and DM1 programs. Vertex will get exclusive worldwide rights to CRISPR Therapeutics’ existing and future intellectual property including foundational CRISPR/Cas9 technology.
Moreover, Vertex will be responsible for all research, development, manufacturing, and commercialization activities and related costs. However, research costs for a specified guide RNA research related to DM1 program will be shared by both the companies. Vertex and CRISPR had signed a four-year research collaboration back to October 2015. Per this agreement, Vertex and CRISPR are developing gene editing therapies for cystic fibrosis and sickle cell disease. In December last year, the companies selected gene therapy, CTX001, to move into clinical development for sickle cell disease and beta thalassemia.
Concurrently, Vertex announced it will acquire privately-held Exonics Therapeutics, which is focused on developing gene editing therapies for DMD and other severe genetic neuromuscular diseases. With the acquisition of Exonics, Vertex will gain intellectual property, technology, and scientific expertise in transformative gene editing therapies. Vertex will pay $245 million upfront to Exonics for acquiring all its outstanding shares and form a wholly-owned subsidiary. Shareholders of Exonics are eligible to receive approximately $1 billion including upfront and potential milestone payments related to the DMD and DM1 programs.
Both the transactions are expected to close in the third quarter of 2019.
CymaBay Crashes on Disappointing NASH Data: Shares of CymaBay plunged 45.5% after the company reported disappointing data from a mid-stage study on lead candidate, seladelpar for the treatment of nonalcoholic steatohepatitis (NASH). 12-week top-line results from the ongoing 52-week phase IIb dose-ranging, paired liver biopsy study of seladelpar showed that treatment with the candidate resulted in minimal reductions in liver fat that were not significant when compared to placebo. While the reductions in markers of liver injury were robust and clinically meaningful, investors were clearly disappointed with the dismal results, given the potential of the NASH space.
Performance
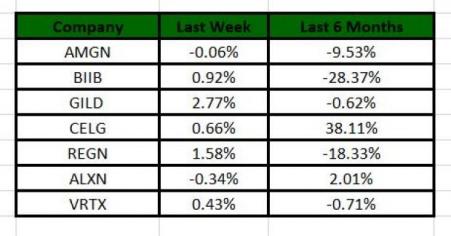
The NASDAQ Biotechnology index lost 0.53% in the last four trading sessions. Among the major biotech giants, Alexion lost 0.34% in the period. Over the past six months, shares of Celgene have surged 38.1% whereas Biogen stock has lost 28.3%. (See the last biotech stock roundup here: Biotech Stock Roundup: AMGN's Nuevolution Buyout, CELG, INCY Drugs' Label Expansion)
What's Next in Biotech?
Stay tuned for more pipeline and regulatory updates.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Insys Therapeutics, Inc. (INSY): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Biogen Inc. (BIIB): Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX): Free Stock Analysis Report
CymaBay Therapeutics Inc. (CBAY): Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP): Free Stock Analysis Report
Original post
Related Articles

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...

The S&P 500 had started to clear resistance, posting new all-time highs before sellers struck with a vengeance. The selling was bad, similar to that seen in December, which...

Myself and others have highlighted how European Equities have been breaking out to new all-time highs on the back of bullish factors such as cheap valuations, monetary tailwinds,...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.