
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Roche To Evaluate Actemra For Severe COVID-19 Pneumonia

Roche Holdings (OTC:RHHBY) announced that it is working with the FDA to initiate a late-stage study to evaluate the safety and efficacy of rheumatoid arthritis drug Actemra/RoActemra (tocilizumab) plus standard of care in hospitalized adult patients with severe COVID-19 pneumonia compared to placebo plus standard of care.
The randomized, double-blind, placebo-controlled phase III study will be conducted in collaboration with the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Health and Human Services Office of the Assistant Secretary for Preparedness and Response (ASPR).
Per Roche, this is the first global study of Actemra/RoActemra in this setting. The study is expected to begin enrolling, with a target of approximately 330 patients globally, including the United States.
The primary and secondary endpoints include clinical status, mortality, mechanical ventilation and intensive care unit (ICU) variables. Patients will be followed for 60 days post-randomization, and an interim analysis will be conducted to look for early evidence of efficacy.
Meanwhile, several independent clinical ongoing studies exploring the efficacy and safety of Actemra/RoActemra for the treatment of patients with COVID-19 pneumonia. The drug has been included in the 7th updated diagnosis and treatment plan for COVID-19 issued by China’s National Health Commission (NHC) on Mar 3, 2020.
Apart from RA, Actemra is approved globally for polyarticular juvenile idiopathic arthritis (pJIA) and in the United States and Europe for systemic juvenile idiopathic arthritis (sJIA) in children two years of age and older.
Currently, there are no FDA-approved treatments for the severe illness caused by SARS-CoV-2. Given the alarming levels of spread and severity, some approved drugs or pipeline candidates are being tested to see if they are effective in treating the infected patients.
Earlier, Roche received FDA Emergency Use Authorisation for the cobas SARS-CoV-2 Test to detect the novel virus that causes the COVID-19 disease.
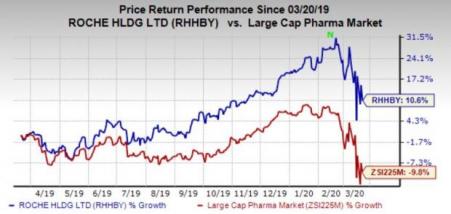
Roche’s stock has gained 10.6% in the past year against the industry’s 9.8% decline.
Quite a few pharma and biotech companies are striving hard to come up with treatments and vaccines to combat this virus.
Regeneron (NASDAQ:REGN) and partner Sanofi (NASDAQ:SNY) have also announced a program to evaluate their RA drug, Kevzara, to treat patients hospitalized with severe infection due to COVID-19. Regeneron also has identified antibodies, which can possibly treat COVID-19. Earlier in the week, Moderna, Inc., (NASDAQ:MRNA) dosed the first participant in the phase I study of mRNA vaccine (mRNA-1273) against the SARS-CoV-2.
Roche currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +50%, +83% and +164% in as little as 2 months. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Sanofi (SNY): Free Stock Analysis Report
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Moderna, Inc. (MRNA): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The fortune of Nvidia (NASDAQ:NVDA) is closely tied to Big Tech hyperscalers. Although the AI/GPU designer didn’t name its largest clients in the latest 10-K filing on Wednesday,...

In a market fraught with uncertainty, investors often seek refuge in defensive-minded stocks that offer stability and resilience. Two such stalwarts, Johnson & Johnson and...

The United States is the largest exporter of liquefied natural gas (LNG), having surpassed Australia and Qatar in 2023. The United States exports an estimated 12.5 billion cubic...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.