
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Regeneron And Sanofi Announce Positive Data On Praluent

Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) and partner Sanofi (NYSE:SNY) announced positive results from the ODYSSEY OUTCOMES trial on Praluent during a late-breaker session at the American College of Cardiology's 67th Annual Scientific Session in Orlando, FL.
The study was evaluating the long-term clinical benefit of Praluent initiation in patients with post-acute coronary syndrome.
The trial met the primary endpoint as results showed that Praluent significantly reduced the risk of major adverse cardiovascular events (“MACE”) in patients with a recent acute coronary syndrome (“ACS”) event such as a heart attack.
Praluent reduced the overall risk of MACE by 15% (HR=0.85, CI: 0.78-0.93, p=0.0003). The MACE composite endpoint includes patients who experienced a heart attack, ischemic stroke, death from coronary heart disease, or unstable angina requiring hospitalization. The results from a pre-specified analysis also showed that the patients with baseline LDL-C levels at or above 100 mg/dL (2.6 mmol/L) experienced a more pronounced effect from Praluent, reducing their risk of MACE by 24% (HR=0.76, CI: 0.65-0.87).
Moreover, no new safety signals emerged from the trial, with injection site reactions experienced more commonly in the Praluent group compared to patients on maximally-tolerated statins alone (3.8% Praluent; 2.1% placebo).
In June 2017, Regeneron and Sanofi announced that two phase IIIb/IV ODYSSEY-DM trials in patients with diabetes met their primary endpoints. While the uptake of the drug hasn’t been encouraging, we note that the cholesterol management market represents huge commercial potential.
We remind investors that Regeneron received a major boost when Praluent became the first PCSK9 inhibitor to be approved (July 2015) in the United States. The drug was also approved in the EU (September 2015). The FDA recently approved the companies' new supplemental Biologics License Application (sBLA) for a once-monthly, 300 mg dose of Praluent(alirocumab) Injection for the treatment of adults with high low-density lipoprotein (LDL) cholesterol. The approval will expand the drug’s dosing options.
Meanwhile, a phase III study evaluating Praluent in homozygous familial hypercholesterolemia was initiated in the fourth quarter of 2017. The sBLA for use of Praluent with apheresis was filed with the FDA, which has set a target action date of Aug 24, 2018. In October 2017, the U.S. Court of Appeals for the Federal Circuit ordered a new trial on the issues of written description and enablement and vacated the permanent injunction in the ongoing PCSK9 litigation.
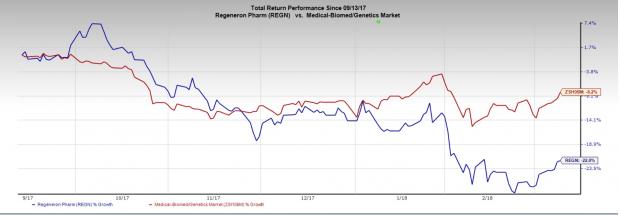
Regeneron’s stock has lost 22% in the last six months compared with the industry’s 8.2% decline.
Regeneron’s fourth-quarter results were impressive as both earnings and sales beat estimates driven by strong Eylea sales. The potential label expansion of Eylea, Dupixent and Praluent will further boost results.
However, although Praluent is the first PCSK9 drug for hypercholesterolemia to get FDA approval, Amgen’s (NASDAQ:AMGN) Repatha is also approved in the United States, the EU and Japan and poses significant competition.
Regeneron and Sanofi announced a reduction in net price for Praluent in alignment with a new value assessment for high-risk patients from the Institute for Clinical and Economic Review. The companies will take a precision medicine approach to address the burden of CV disease, focusing efforts on high-risk patients most vulnerable to future CV events, such as those who have suffered a previous coronary event and are unable to reduce their LDL cholesterol (LDL-C) below 100 mg/dL despite maximally-tolerated statin therapy.
Zacks Rank & Another Stock to Consider
Regeneron sports a Zacks Rank #1 (Strong Buy). You can see the the complete list of today’s Zacks #1 Rank stocks here.
Another top-ranked stock in the healthcare sector is Ligand Pharmaceuticals (NASDAQ:LGND) which carries a Zacks Rank #2 (Buy).
Ligand’s earnings per share estimates have moved up $3.78 to $4.15 from $4.75 to $5.75 for 2018 and 2019 respectively over the last 30 days. The company delivered positive earnings surprises in three of the trailing four quarters, with an average beat of 24.88%.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Sanofi (SNY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The fortune of Nvidia (NASDAQ:NVDA) is closely tied to Big Tech hyperscalers. Although the AI/GPU designer didn’t name its largest clients in the latest 10-K filing on Wednesday,...

In a market fraught with uncertainty, investors often seek refuge in defensive-minded stocks that offer stability and resilience. Two such stalwarts, Johnson & Johnson and...

The United States is the largest exporter of liquefied natural gas (LNG), having surpassed Australia and Qatar in 2023. The United States exports an estimated 12.5 billion cubic...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.