
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Pfizer's Eczema Drug & Pneumococcal Vaccine Succeed In Study

Pfizer Inc. (NYSE:PFE) announced positive top-line data from two separate phase III studies on its atopic dermatitis (also called eczema) candidate, abrocitinib, and 20-valent pneumococcal conjugate vaccine (20vPnC) candidate.
A phase III study — JADE COMPARE — evaluating two doses of oral JAK inhibitor, abrocitinib, in patients with moderate-to-severe atopic dermatitis and on background topical therapy met its co-primary endpoints. Statistically higher percentage of patients receiving abrocitinib witnessed improvement in clear skin from baseline compared to placebo.
The JADE COMPARE study also had a control arm evaluating Sanofi (NASDAQ:SNY) /Regeneron’s (NASDAQ:REGN) blockbuster drug, Dupixent (dupilumab). Data showed that treatment with higher dosage of abrocitinib achieved clinically significant reduction in itch compared to Dupixent.
Last year, Pfizer had announced positive top-line data from two phase III studies evaluating two doses of abrocitinib in similar patient population. Top-line data from these studies showed that by week 12, both doses of abrocitinib led to a statistically significantly higher percentage of patients achieving improvement in clear skin. Both doses were well tolerated in all studies.
The company plans to file regulatory application seeking approval for abrocitinib based on data from these three studies later this year.
Meanwhile, we note that several other companies are also developing their drugs for treating atopic dermatitis, which includes Lilly (NYSE:LLY) Olumiant and AbbVie’s Rinvoq.
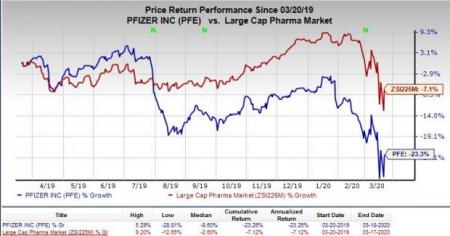
Shares of Pfizer have lost 23.3% in the past year compared with the industry's decrease of 7.1%.
The company evaluated its 20vPnC candidate in a phase III study in patients who have not received any vaccination against pneumococcal disease. Data showed that 20vPnC candidate achieved non-inferiority in immunogenicity compared to Pfizer’s popular 13-valent pneumococcal conjugate vaccine, Prevnar 13 for all common 13 serotypes. The 20vPnC candidate also achieved non-inferiority compared to a licensed pneumococcal polysaccharide vaccine for six of the new seven serotypes. However, one of the new seven serotypes failed to show non inferiority to licensed vaccines by a small margin. Data from the study are expected to meet licensure criteria for 20vPnC vaccine candidate. The company expects to file a new drug application for the vaccine candidate this year.
Pfizer has completed two other phase III studies evaluating the 20vPnC candidate. Data from these studies will be announced over the next few months.
Zacks Rank
Pfizer currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +50%, +83% and +164% in as little as 2 months. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Sanofi (SNY): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Original post
Related Articles

The fortune of Nvidia (NASDAQ:NVDA) is closely tied to Big Tech hyperscalers. Although the AI/GPU designer didn’t name its largest clients in the latest 10-K filing on Wednesday,...

In a market fraught with uncertainty, investors often seek refuge in defensive-minded stocks that offer stability and resilience. Two such stalwarts, Johnson & Johnson and...

The United States is the largest exporter of liquefied natural gas (LNG), having surpassed Australia and Qatar in 2023. The United States exports an estimated 12.5 billion cubic...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.