
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Lilly's Olumiant Gets Breakthrough Therapy Tag For Hair Loss

Eli Lilly (NYSE:LLY) announced that the FDA has granted Breakthrough Therapy designation to its oral JAK inhibitor, Olumiant (baricitinib) as a treatment for alopecia areata (“AA”), an autoimmune disorder that may lead to unpredictable hair loss from the scalp, face and other areas of the body. The drug is currently in late-stage development as a treatment for AA.
FDA’s Breakthrough Therapy designation is granted to speed up development and review of drugs that target serious or life-threatening conditions. The designation also indicates that Olumiant can be eligible for accelerated approval and priority review depending upon certain criteria. The designation also reinforces the potential of Olumiant to be the first FDA-approved treatment for AA.
The FDA granted the designation based on positive data from the phase II portion of the phase II/III study – BRAVE-AA1. The company started evaluating Olumiant in the phase III portion of the BRAVE-AA1 study based on interim phase II result. It is also evaluating the drug in a separate phase III study — BRAVE-AA2 — in a similar patient population.
Please note that Olumiant is already approved for treating moderately-to-severely active rheumatoid arthritis. Successful development as a treatment for AA patients will further boost its prospect.
However, competition may rise going forward as Pfizer (NYSE:PFE) is also developing its oral JAK inhibitor for treating AA in a pivotal study.
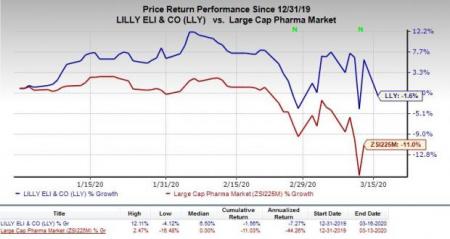
So far this year, shares of Lilly have declined 1.6% compared with the industry's decrease of 11%.
Apart from AA, the company is also focused on expanding the label of Olumiant to include other auto-immune indications. The drug is under review in Europe and Japan for atopic dermatitis, a type of eczema, and a regulatory application will be filed seeking approval for the same in the United States later in 2020. A phase III study is also evaluating the drug in patients with systemic lupus erythematosus.
Baricitinib is being co-developed by Lilly and Incyte (NASDAQ:INCY) under an exclusive global license and collaboration agreement inked in December 2009.
Zacks Rank & Stock to Consider
Lilly currently carries a Zacks Rank #3 (Hold).
Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) is better-ranked stock, sporting a Zacks Rank #1 (Strong Buy). Regeneron’s earnings estimates for 2020 have gone up from $28.31 to $29.18 and from $28.93 to $30.97 for 2021 over the past 30 days. Regeneron’s stock has returned 17.4% so far in 2020
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The fortune of Nvidia (NASDAQ:NVDA) is closely tied to Big Tech hyperscalers. Although the AI/GPU designer didn’t name its largest clients in the latest 10-K filing on Wednesday,...

In a market fraught with uncertainty, investors often seek refuge in defensive-minded stocks that offer stability and resilience. Two such stalwarts, Johnson & Johnson and...

The United States is the largest exporter of liquefied natural gas (LNG), having surpassed Australia and Qatar in 2023. The United States exports an estimated 12.5 billion cubic...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.