
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Hologic (HOLX) Gets EUA For Its Coronavirus Detecting Test

Hologic, Inc. (NASDAQ:HOLX) announced the receipt of the FDA’s Emergency Use Authorization (“EUA”) for its latest molecular diagnostic test, Panther Fusion SARS-CoV-2 assay. Notably, the test detects SARS-CoV-2.
Per Hologic, it is the first company to receive support for developing the coronavirus detection test from the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (“BARDA”).
With this, Hologic aims to strengthen the foothold in the global Molecular Diagnostics market.
A Peek Into Panther Fusion System
Panther Fusion system is a fully automated high-throughput molecular diagnostic platform, which is widely used across the United States. Notably, the Panther Fusion SARS-CoV-2 assay is a real-time polymerase chain reaction (RT-PCR) in vitro diagnostic test, which is intended to be used for the qualitative detection of RNA from the SARS-CoV-2. The test is done after the collection of swab specimens from patients, who meet the COVID-19 clinical and/or epidemiological criteria.
Regulatory Status of Panther Fusion System
The Panther Fusion SARS-CoV-2 assay has not been cleared or approved by the FDA yet. It has only been authorized by the FDA under a EUA for use by laboratories certified under the Clinical Laboratory Improvement Amendments (“CLIA”) to perform high-complexity tests.
The test has been permitted only to be used to detect RNA from SARS-CoV-2 and the diagnosis of SARS-CoV-2 infection. Further, the authorization is only valid for the duration of the declaration that circumstances exist, justifying the authorization of the emergency use of in-vitro diagnostics, for the detection of SARS-CoV-2.
Significance of the Approval
Hologic’s Panther Fusion system can be availed by hospitals, public health organizations and reference laboratories to provide faster results. Notably, the test to detect SARS-CoV-2 can be performed from the same patient sample and collection vial, which is already being used to detect other common respiratory viruses whose symptoms overlap with COVID-19. This can be done through the Panther Fusion system, thus, boosting efficiency and increasing clinical insight.
Another significant advantage of the use of the system is that patients’ samples can be loaded onto the Panther Fusion system as soon as they arrive in the laboratory. The capability of faster loading of the sample, known as random access, is expected to speed up the testing, and improve efficiency and workflow by the instrument’s high throughput and quick turnaround time.
Industry Prospects
Per a report published on Grand View Research, the global molecular diagnostics market size was valued at $9.2 billion in 2019 and is expected to reach $18.2 billion by 2027, witnessing a CAGR of 9% between 2020 and 2027. Factors like technological advancements in molecular diagnostics as well as the rising prevalence of infectious diseases are likely to drive the market.
Given the global condition of the market amid the coronavirus outbreak, Hologic’s efforts to develop, validate and get the EUA within two months is noteworthy.
Recent Developments in Molecular Diagnostics
Of late, Hologic has been concentrating on helping contain the outbreak.
Per the company, it ramped up the production capacity to provide a greater number of SARS-CoV-2 tests in March. Further, it expects to provide around 600,000 SARS-CoV-2 tests a month from April onward. Notably, the company confirmed making additional investments to further increase its production capacity.
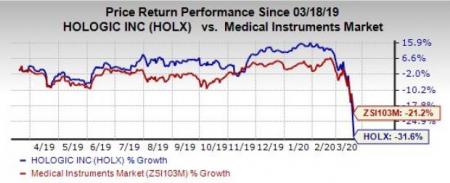
Price Performance
Shares of Hologic have dipped 31.6% in the past year compared with the industry’s 21.2% decline.
Zacks Rank & Key Picks
Currently, the company carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the broader medical space are ResMed Inc. (NYSE:RMD) , Medtronic plc (NYSE:MDT) and Hill-Rom Holdings, Inc. (NYSE:HRC) .
ResMed has a projected long-term earnings growth rate of 14.4%. It currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Medtronic’s long-term earnings growth rate is estimated at 7.4%. The company presently has a Zacks Rank #2.
Hill-Rom’s long-term earnings growth rate is estimated at 11.1%. It currently carries a Zacks Rank #2.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Medtronic PLC (MDT): Free Stock Analysis Report
Hologic, Inc. (HOLX): Free Stock Analysis Report
ResMed Inc. (RMD): Free Stock Analysis Report
Hill-Rom Holdings, Inc. (HRC): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The fortune of Nvidia (NASDAQ:NVDA) is closely tied to Big Tech hyperscalers. Although the AI/GPU designer didn’t name its largest clients in the latest 10-K filing on Wednesday,...

In a market fraught with uncertainty, investors often seek refuge in defensive-minded stocks that offer stability and resilience. Two such stalwarts, Johnson & Johnson and...

The United States is the largest exporter of liquefied natural gas (LNG), having surpassed Australia and Qatar in 2023. The United States exports an estimated 12.5 billion cubic...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.