Bristol-Myers Squibb Company (NYSE:BMY) has announced that its supplemental Biologics License Application (sBLA) for its PD-1 immune checkpoint inhibitor, Opdivo (nivolumab), in combination with Yervoy (ipilimumab) has been accepted by the FDA under priority review.
The company seeks an approval for label expansion of the combination therapy for addressing patients, previously treated with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer (mCRC). A response from the regulatory body is awaited on Jul 10, 2018.
The Opdivo/Yervoy combo regimen has also been granted a Breakthrough Therapy Designation by the FDA for the aforementioned indication.
Notably, Opdivo acquires a nod in several countries including the United States, the EU and Japan for several cancer indications.
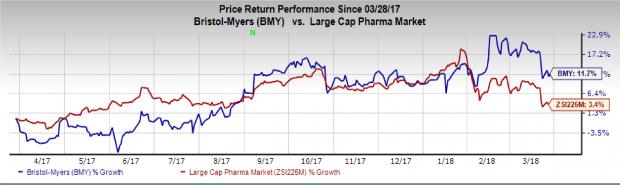
Shares of the company have rallied 11.7% in the past year, outperforming the industry’s gain of 3.4%.
The acceptance of the application was based on positive data from Phase II CheckMate-142 study, announced in January 2018, evaluating the combination therapy for the given indication.
Results from the study showed that the primary endpoint of objective response rate per investigator assessment was 55% with a median follow-up of 13.4 months. The overall survival rate at one year was 85% and the median OS was not yet reached. The outcomes showed that Opdivo plus Yervoy provide a durable clinical relief in patients with dMMR or MSI-H metastatic colorectal cancer.
The data demonstrated statistically significant and clinically meaningful improvements in key patient reports including symptoms, functioning and quality of life.
Significantly, this combination procedure is also under assessment in a phase III, Checkmate-227 trial as a first-line treatment for non-small cell lung cancer (NSCLC). Last month, Bristol Myers announced positive data from the program, reflecting superior progression-free survival in first-line NSCLC patients compared with chemotherapy. This is the first study to examine combination regimes of two immune-oncology drugs in first line NSCLC patients with high tumor mutation burden.
Per the company’s press release, colorectal is one of the most common cancer types and the third leading cause for terminal disease-related deaths in the United States. Approximately, 140,000 new cases of CRC are expected to be diagnosed annually in the country. Moreover, patients with MSI-H or dMMR metastatic CRC have mild possibility to benefit from conventional chemotherapy treatment. Hence, approval of this combo treatment will provide the company with access to a huge patient-population base, in need of an alternative treatment option to apprehend the disease.
However, Bristol-Myers faces a fierce competitive pressure in the immuno-oncology space. In fact, Opdivo battles against stiff rivalry from Merck’s (NYSE:MRK) anti-PD-1 immunotherapy Keytruda and Roche’s Tecentriq. Merck’s Keytruda is also being analyzed in a phase III study for CRC. Hence, label expansion of Opdivo/Yervoy combination will lend the company a wider population base of patients for Opdivo.
A few approved drugs to treat patients with CRC are Lilly’s (NYSE:LLY) Cyramza, Roche’s (OTC:RHHBY) Avastin and Amgen’s Vectibix.
Zacks Rank
Bristol-Myers carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Original post
Zacks Investment Research