
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Endocyte (ECYT) Reports Wider-Than-Estimated Loss In Q4

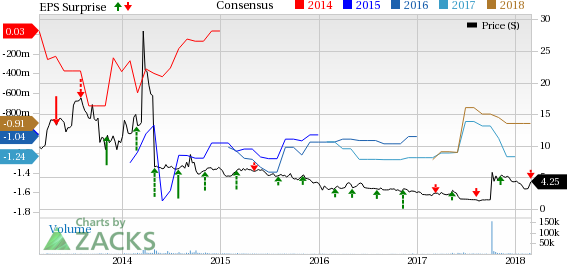
Endocyte, Inc. (NASDAQ:ECYT) incurred a loss of 18 cents per share in fourth-quarter 2017 wider than the Zacks Consensus Estimate of a loss of 16 cents. The figure was wider than the year-ago loss of 26 cents.
In September 2017, the company acquired exclusive worldwide rights to develop and commercialize PSMA-617 including the product candidate known as 177Lu-PSMA-617 from ABX GmbH. 177Lu-PSMA-617 is a first-in-class radioligand therapeutic (RLT) that targets prostate-specific membrane antigen (“PSMA”).
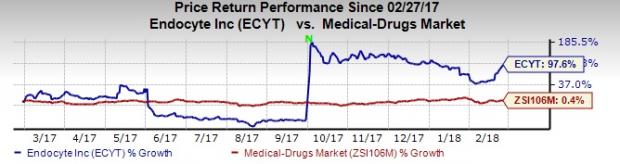
Over a year, Endocyte’s shares have rallied 97.6%, outperforming the industry’s gain of 0.4%.
Quarterly Details
Research and development (R&D) expenses declined 37.4% year over year to $5.2 million courtesy of the workforce reduction as part of the company’s restructuring plan, lower trial and manufacturing costs for EC1456, lower pre-clinical general research costs and a decrease in manufacturing expense for EC1169.
General and administrative expenses increased 19.8% year over year at $3.7 million due to higher legal and professional costs and an increase in compensation expense, including stock compensation expense.
During the quarter, the company finalized a phase III VISION trial design for177Lu-PSMA-617 following a successful End of Phase II meeting with the FDA. The trial will include two interim assessments of efficacy, which could potentially lead to an early approval for 177Lu-PSMA-617. The VISION trial is a phase III study of 177Lu-PSMA-617 for the treatment of patients with PSMA-positive metastatic castration-resistant prostate cancer, who have received at least one novel androgen axis drug and at least one taxane regimen. The primary endpoint of the study will be overall survival. Enrollment of the trial is expected to begin in second-quarter 2018 and is expected to be completed within 18-24 months.
Endocyte also entered into an agreement with ITM Isotopen Technologien München AG (“ITM”), a specialized radiopharmaceutical company, where the latter will provide clinical supply of no-carrier-added Lutetium for the manufacturing of 177Lu-PSMA-617.
In order to ensure the success of its VISION study, Endocyte added clinical trial professionals to the team. Caryn Barnett and Theresa Bruce both seasoned leaders with strong track records of executing late stage oncology development programs joined the company’s team.
Endocyte also finalized plans for the clinical development of adaptor-controlled CAR T-cell therapy in patients with osteosarcoma in fourth-quarter 2018.
2017 Results
For 2017, the company reported a loss of $1.25 per share wider than the loss of $1.04 in 2016. The loss is wider than the Zacks Consensus loss estimate of $1.24.
Endocyte, Inc. Price, Consensus and EPS Surprise
Zacks Rank & Stocks to Consider
Endocyte carries a Zacks Rank #3 (Hold).
A few better-ranked stocks from the same space are Regeneron (NASDAQ:REGN) , Exelixis (NASDAQ:EXEL) and Enanta Pharma (NASDAQ:ENTA) . While Regeneronsports a Zacks Rank #1 (Strong Buy), Exelixis and Enanta Pharma carry a Zacks Rank #2 (Buy), each. You can see the complete list of today’s Zacks #1 Rank stocks here.
Regeneron’s earnings per share estimates have moved up from $17.13 to $18.65 and from $20.38 to $21.56 for 2018 and 2019 respectively in the last 30 days. The company pulled off a positive earnings surprise in three of the last four quarters, with an average beat of 9.15%.
Exelixis’ earnings per share estimates have moved up from 73 cents to 77 cents for 2018 in the last 60 days. The company delivered a positive earnings surprise in the last four quarters, with an average beat of 572.92%. Share price of the company moved up 27.8% over a year.
Enanta Pharma delivered a positive earnings surprise in three of the last four quarters, with an average beat of 373.1%. Share price of the company surged 166.8% over a year
Zacks Top 10 Stocks for 2018
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-hold tickers for the entirety of 2018?
Last year's 2017 Zacks Top 10 Stocks portfolio produced double-digit winners, including FMC Corp (NYSE:FMC). and VMware which racked up stellar gains of +67.9% and +61%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys.
Access Zacks Top 10 Stocks for 2018 today >>
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Endocyte, Inc. (ECYT): Free Stock Analysis Report
Enanta Pharmaceuticals, Inc. (ENTA): Free Stock Analysis Report
Original post
Related Articles

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...

The S&P 500 had started to clear resistance, posting new all-time highs before sellers struck with a vengeance. The selling was bad, similar to that seen in December, which...

Myself and others have highlighted how European Equities have been breaking out to new all-time highs on the back of bullish factors such as cheap valuations, monetary tailwinds,...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.