
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Clovis' SNDA For Rubraca Gets Priority Review In The U.S.

Clovis Oncology (NASDAQ:CLVS) announced the FDA has granted priority review to its supplemental new drug application (sNDA) looking for label expansion of its marketed drug, Rubraca (rucaparib).
The sNDA is looking to expand Rubraca’s label as a maintenance treatment for patients with platinum-sensitive recurrent ovarian cancer regardless of a patient’s BRCA mutation status. With the FDA granting priority review, a response from the regulatory body is expected in Apr 6, 2018. Approval of the drug for this expanded indication will provide the company access to a wider population base of patients with ovarian cancer.
Notably, Rubraca is a PARP inhibitor, having received an accelerated approval in the United States in December 2016 for advanced ovarian cancer patients with deleterious BRCA mutation, previously treated with two or more chemotherapies.
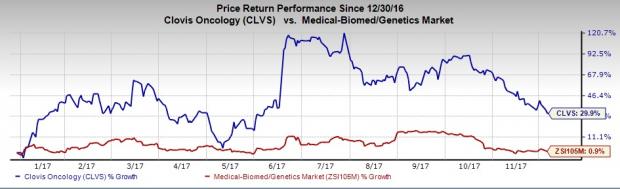
Clovis’ shares are up almost 29.9% so far this year, significantly outperforming the industry’s 0.9% rally during the period.
The sNDA was submitted in October, based on promising data from the confirmatory phase III study, ARIEL 3, announced in June. Rubraca demonstrated a meaningful impact in delaying disease recurrence in advanced ovarian cancer patients, who are either BRCA mutant or HRD positive.
We remind investors that Rubraca as a monotherapy is also under review in the EU for treating advanced ovarian cancer. An opinion is expected later this month.
Clovis is also planning to file a marketing authorization application in early 2018 for maintenance treatment in the EU following the potential approval as monotherapy.
A potential approval in the EU and the label expansion in the United States are expected to boost the drug’s potential. It has generated sales of nearly $38.5 million in the first nine months of 2017 post its launch in December last year.
However, Clovis’ Rubraca is facing competition from other PARP inhibitors including Tesaro, Inc.'s (NASDAQ:TSRO) Zejula and AstraZeneca's (NYSE:AZN) Lynparza as both these drugs are also approved to treat ovarian cancer irrespective of the BRCA-mutation.
Meanwhile, the second phase III confirmatory study, ARIEL4, is evaluating Rubraca compared with chemotherapy on patients, who have failed two prior lines of therapy. Clovis is also looking to expand Rubraca’s label into additional indications like prostrate, breast and pancreatic cancers among others either as monotherapy or in combination with other agents.
Other ongoing clinical analyses include phase II TRITON2 on prostate cancer and phase III TRITON3, a Tecentriq-Rubraca combination study on gynecologic cancers, sponsored by Roche (OTC:RHHBY) .
Zacks Rank
Cloviscarries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
TESARO, Inc. (TSRO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The fortune of Nvidia (NASDAQ:NVDA) is closely tied to Big Tech hyperscalers. Although the AI/GPU designer didn’t name its largest clients in the latest 10-K filing on Wednesday,...

In a market fraught with uncertainty, investors often seek refuge in defensive-minded stocks that offer stability and resilience. Two such stalwarts, Johnson & Johnson and...

The United States is the largest exporter of liquefied natural gas (LNG), having surpassed Australia and Qatar in 2023. The United States exports an estimated 12.5 billion cubic...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.