
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Cancer Space Update: FDA Approves Label Expansion Of 4 Drugs

After a busy last week filled with clinical trial data presentation at the annual meeting of the American Society of Hematology, the cancer space saw regulatory approvals for label expansion of four drugs this week.
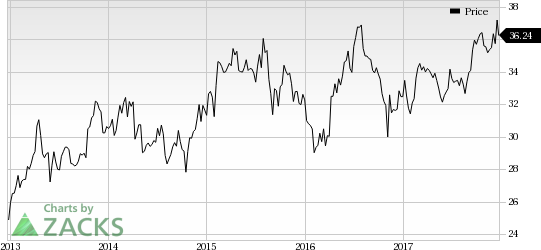
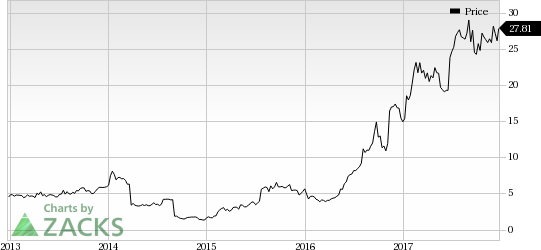
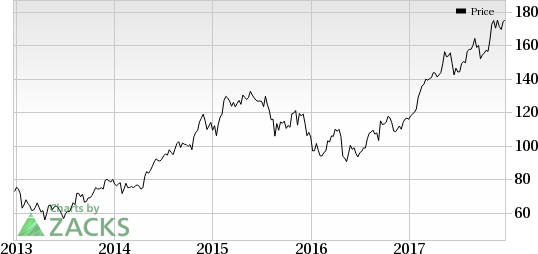
Roche Holding (SIX:ROG) AG’s (OTC:RHHBY) breast cancer drug, Perjeta, received FDA nod for its label expansion in post-surgery breast cancer with high risk of recurrence. Bristol-Myers Squibb Company’s (NYSE:BMY) blockbuster PD-1 inhibitor drug, Opdivo, received approval for intravenous administration in patients with completely resected melanoma with lymph node involvement or metastatic disease in the United States. Meanwhile, Pfizer, Inc.’s (NYSE:PFE) Bosulif has been approved for treating Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (“CML”) in first-line setting. Exelixis, Inc. (NASDAQ:EXEL) also announced the approval of Cabometyx’s label expansion in previously untreated advanced renal cell carcinoma (“RCC”).
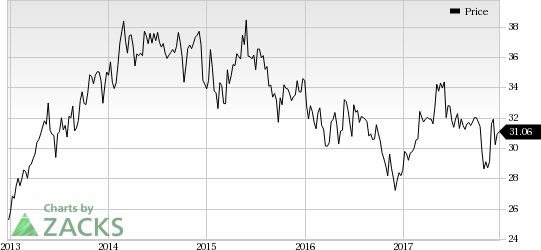
However, Celgene Corporation (NASDAQ:CELG) suffered a setback when its key drug, Revlimid, failed in a phase III study evaluating it as a maintenance therapy in treatment-naïve follicular lymphoma patients.
Meanwhile, AstraZeneca (NYSE:AZN) announced the acceptance of a supplemental New Drug Application (sNDA) by the FDA that sought label expansion of Tagrisso to include first-line treatment of adult patients with locally-advanced or metastatic non-small cell lung cancer (“NSCLC”) whose tumors have EGFR mutations. The sNDA was also granted priority review, suggesting speedy review with a decision in the next six months.
Let’s see the major news in details.
Roche's Perjeta Gets Approval in Post-Surgery Breast Cancer: The FDA approved Perjeta in combination with Herceptin and chemotherapy as an adjuvant therapy for HER2-positive early breast cancer at high risk of recurrence. The drug regimen significantly reduced the risk of disease recurrence or death compared to Herceptin and chemotherapy alone. (Read more: Roche's Perjeta Gets FDA Nod for Post Surgery Breast Cancer)
Also, during the week, Roche entered into an agreement to acquire U.S. based biotech company, Ignyta for $1.7 billion to boost its oncology portfolio. Ignyta is focused on developing therapy for cancer with rare mutations. Ignyta’s lead candidate, entrectinib, is currently in a phase II study in patients with NSCLC.
Pfizer’s Bosulif Approved as First-line Therapy Leukemia: Bosulif’s label expansion in first line setting for patients with chronic phase Ph+ CML was approved by the FDA. The drug is already approved in second or later settings in this indication. (Read more: Pfizer's Leukemia Drug Gets FDA Nod in First-Line Setting)
The FDA has also granted Breakthrough Therapy Designation to its combination therapy of Bavencio and Inlyta, which is being investigated in patients with advanced RCC as first-line treatment. The combination therapy is currently being evaluated in a phase Ib study.
Exelixis' Cabometyx Approved in First-Line Kidney Cancer: Exelixis’s RCC drug, Cabometyx, was granted label expansion in first-line setting. The FDA approved the label expansion based on data from phase II CABOSUN study, which demonstrated that Cabometyx was superior to Pfizer’s Sutent in treating first line kidney cancer. (Read more: Exelixis' Cabometyx Gets FDA Nod for First-Line Kidney Cancer)
Exelixis carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Celgene’s Revlimid Failed in Phase III Lymphoma Study: Celgene was down following the news of failure of a phase III Revlimid study. The study evaluated Revlimid in combination with Roche’s Rituxan as a maintenance therapy for treatment-naïve patients with follicular lymphoma. The combination failed to achieve superiority in complete response and progression-free survival over standard of care with Rituxan plus chemotherapy.
Revlimid is the primary growth driver for Celgene and a label expansion in lymphoma would have further boosted its potential.
Bristol-Myers’ Opdivo Approved for Intravenous Use Metastatic Melanoma: The FDA approved the intravenous administration of the PD1 inhibitor, Opdivo, in adjuvant treatment of post-surgery patients with melanoma, which involves lymph nodes or are metatstatic. The approval was based on data from phase III CheckMate-238 study. Data from the study has demonstrated that Opdivo has significantly improved recurrence-free survival compared to Yervoy. Opdivo is the first drug to receive approval as an adjuvant treatment in this indication.
Bristol-Myers has also announced that the Committee for Medicinal Products for Human Use has recommended the approval of Yervoy in Europe for treating pediatric patients of 12 years and older with unresectable or metastatic melanoma. (Read more: Bristol-Myers Yervoy Gets Positive CHMP Opinion for Melanoma)
Meanwhile, BioLineRx initiated phase III GENESIS study to evaluate its lead pipeline candidate, BL-8040. The study will investigate the mobilization of stem cells in patients undergoing autologous transplant for multiple myeloma. Juno Therapeutics (NASDAQ:JUNO) announced a partnership with Thermo Fisher Scientific (NYSE:TMO) to use the latter’s Cell Therapy Systems activation reagents to develop its CAR-T therapy pipeline.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Roche Holding AG (RHHBY): Free Stock Analysis Report
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Pfizer, Inc. (PFE): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Juno Therapeutics, Inc. (JUNO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...

The S&P 500 had started to clear resistance, posting new all-time highs before sellers struck with a vengeance. The selling was bad, similar to that seen in December, which...

Myself and others have highlighted how European Equities have been breaking out to new all-time highs on the back of bullish factors such as cheap valuations, monetary tailwinds,...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.