
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Biotech Stock Roundup: HOTH & IMV To Develop Coronavirus Vaccines, & More

While the prime focus is currently on coronavirus treatments and vaccines for the biotech sector, a few other updates were also out this week. A slew of biotechs is evaluating their pipeline drugs and vaccines for the treatment of COVID-19 and updates from these companies will drive their share prices.
Recap of the Week’s Most Important Stories:
Gilead's HCV Drug Epclusa Gets FDA Nod for Expanded Age Group: Gilead Sciences (NASDAQ:GILD) announced that the FDA has approved the supplemental new drug application (sNDA) to update its hepatitis C infection (HCV) drug, Epclusa’s label. The sNDA sought approval of Epclusa for the treatment of chronic HCV in pediatric patients aged six years and above or weighing at least 17 kg, regardless of HCV genotype or liver disease severity. The approval was based on data from the open-label phase II Study 1143 trial. The safety profile of Epclusa in the pediatric patient population was generally consistent with what was observed in adults.
Hoth Surges on Collaboration With Voltron: Shares of clinical-stage biopharmaceutical company, Hoth Therapeutics, Inc. (NASDAQ:HOTH) , surged after it announced that it has reached an agreement with Voltron Therapeutics, Inc. to form a joint venture for a Self-Assembling Vaccine (SAV) for the potential prevention from the coronavirus (COVID-19). The joint entity will be called HaloVax. Based on VaxCelerate, this SAV platform was exclusively licensed by Voltron from the Vaccine and Immunotherapy Center (VIC) at Massachusetts General Hospital (MGH). Both companies will jointly explore and develop this SAV technology as a means to help patients at risk of being infected with COVID-19. The vaccine focuses on both DNA and internal/external mutated proteins, providing the immune system with more potential targets to attack.
Per the agreement, Hoth shall be granted the right to receive single-digit royalties from the sale of any products developed. It shall also have the right to acquire up to a 30% equity interest in HaloVax.
Hoth currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Milestone Plunges on Study Failure: Milestone Pharmaceuticals Inc. (NASDAQ:MIST) plunged after it announced the failure of its late-stage study on the investigational new drug, etripamil nasal spray, the company's novel short-acting calcium channel blocker, in patients with paroxysmal supraventricular tachycardia (PSVT). The phase III, multicenter, randomized, double-blind, placebo-controlled NODE-301 trial missed the primary endpoint of mean time to conversion of supraventricular tachycardia (SVT) to sinus rhythm (SR) over a five-hour period following dosing.
Novavax Surges on Successful Flu Study Results: Novavax (NASDAQ:NVAX) surged after it announced that NanoFlu achieved all primary endpoints in a phase III study. NanoFlu is the company’s recombinant quadrivalent seasonal influenza vaccine candidate with its proprietary Matrix-M adjuvant, indicated for use in adults aged 65 years and older. The study evaluated the immunogenicity and safety of NanoFlu compared to FluzoneQuadrivalent, a U.S.-licensed quadrivalent influenza vaccine, using the FDA’s criteria for accelerated approval of seasonal influenza vaccines. The trial’s primary objectives were to demonstrate non-inferior immunogenicity of NanoFlu compared to Fluzone Quadrivalent using the day 28 ratio of geometric mean titers (GMT) and the difference in seroconversion rates (SCR), as well as the overall safety of NanoFlu. Immunogenicity was measured by hemagglutination inhibition (HAI) assays using egg-derived reagents. NanoFlu achieved the primary endpoints, GMT and SCR, for all four strains included in the vaccine.
IMV to Develop Vaccine Candidate Against COVID-19: IMV Inc. (TSX:IMV) announced that it plans to start the clinical development of a DPX-based vaccine candidate for the treatment of the novel coronavirus disease. The DPX-COVID-19 vaccine will be made using its DPX technology platform. The company plans to develop this vaccine candidate and begin its phase I clinical study in collaboration with lead investigators. Per the company, the aim of this development program is to establish the clinical safety and immunogenicity of a vaccine candidate based on IMV’s lipid-based delivery platform, DPX technology, and incorporate peptides targeting novel epitopes from the coronavirus strain.
Performance
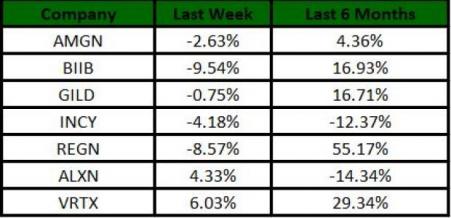
The Nasdaq Biotechnology index gained 2.06% in the last five trading sessions. Among the biotech giants, Vertex (NASDAQ:VRTX) gained 6.03% during this period. Over the past six months, shares of Regeneron have gained 55.17%. (See the last biotech stock roundup here: Biotech Stock Roundup: MRNA & REGN Gain on Coronavirus Treatment Updates & More)
Stay tuned for more pipeline updates, with a focus on treatments for the novel COVID-19.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
IMV INC (IMV): Free Stock Analysis Report
Hoth Therapeutics, Inc. (HOTH): Free Stock Analysis Report
Milestone Pharmaceuticals Inc. (MIST): Free Stock Analysis Report
Original post
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.