
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Glaxo's Dovato Wins EU Nod, RA Candidate Enters Phase III

GlaxoSmithKline plc’s (NYSE:GSK) HIV subsidiary, ViiV Healthcare, announced that the European Commission has granted a marketing authorization to Dovato, its once-daily, single-tablet two-drug HIV regimen. The approval in the EU is for the treatment of HIV-1 infection in adults/adolescent patients, aged above 12 years and weighing minimum 40 kg with no known resistance to integrase inhibitors or lamivudine.
ViiV Healthcare is majorly owned by Glaxo and Pfizer (NYSE:PFE) .
Dovato is a fixed-dose combination of Tivicay (dolutegravir - 50 mg) and lamivudine (300 mg) and was approved by the FDA in this April.
The EU approval was based on data from the GEMINI 1&2 studies, which evaluated more than 1,400 patients with HIV-1 infection. Data from the studies showed that after 48 weeks, treatment with the two-drug regimen of Dovato demonstrated non-inferior efficacy as compared to a three-drug regimen for treatment-naïve, HIV-1 patients. This means, a two-drug HIV regimen can control the intensity of HIV in treatment naïve patients as effectively as a three-drug regimen, thereby exposing the newly diagnosed patients to fewer drugs at the start of their treatment for the first time.
Following this nod, patients can begin treatment with Dovato as the first once-daily two-drug regimen knowing that efficacy is non-inferior to the standard three-drug regimen for the given patient population.
Shares of Glaxo have rallied 8.1% so far this year, outperforming the industry’s rise of 3.7%.
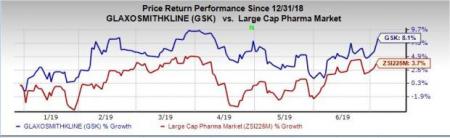
HIV is a key therapeutic area for Glaxo with successful dolutegravir-based regimens, namely Tivicay, Triumeq and Juluca in its portfolio. HIV sales totaled £1.1 billion in the first quarter of 2019. The metric increased 4% at CER on the back of 7% growth in dolutegravir franchise (Triumeq and Tivicay and Juluca), which partly offset lower sales of the established HIV products.
In a separate press release, Glaxo announced that it has started the phase III program on its anti GM-CSF antibody, otilimab,for the treatment of patients with moderate-to-severe rheumatoid arthritis (RA), who do not respond to disease modifying antirheumatic drugs (DMARD) or any such targeted therapies.
The phase III ContRAst study will also include head-to-head comparison of otilimab with Pfizer's Xeljanz (tofacitinib) and Regeneron Pharmaceuticals (NASDAQ:REGN) / Sanofi (PA:SASY)'s (NASDAQ:SNY) Kevzara (sarilumab) across all pivotal studies.
The primary endpoint of these analyses will assess the proportion of patients achieving the American College of Rheumatology criteria (ACR20) at 12-week treatment compared with placebo.
The study will be conducted across a broad range of difficult-to-treat patients. The initiation of the phase III program was based on encouraging data from the phase II BAROQUE study, which showed striking clinical improvements in patients treated with otilimab.
Zacks Rank
Glaxo currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Pfizer Inc. (PFE): Free Stock Analysis Report
Sanofi (SNY): Free Stock Analysis Report
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Since the Robotaxi event on October 11th, Tesla (NASDAQ:TSLA) stock is up 38%, currently priced at $291.60 per share This is a return to the early November 2024 price level. But...

The Q4 2024 earnings season tapers off from here, with S&P 500® EPS growth surpassing 17%, the highest in 3 years Large cap outlier earnings dates this week include:...

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.