
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

TESARO (TSRO) Ovarian Cancer Drug Zejula Gets Approval In EU

TESARO, Inc. (NASDAQ:TSRO) announced that the European Commission has granted marketing authorization to its PARP inhibitor, Zejula, as monotherapy for the maintenance treatment of women with recurrent ovarian cancer. The drug is the first approved PARP inhibitor in Europe, which does not require BRCA mutation or other biomarker testing.
The marketing approval in EU has boosted prospects of Zejula, which is already marketed in the United States. The approval in EU was expected as the drug was recommended by the Committee for Medicinal Products for Human Use in September.
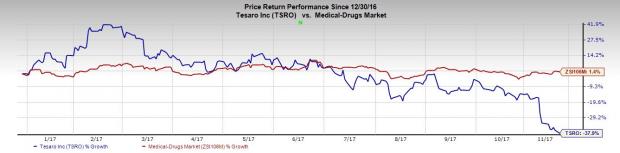
A look at TESARO’s share price movement so far this year shows that the company has underperformed the industry. While the stock fell 37.9%, the industry registered a gain of 1.4%.
The approval was based on data from an international phase III study, ENGOT-OV16/NOVA, which showed clinically meaningful increase in progression-free survival. . The study evaluated the drug in recurrent ovarian cancer patients who had achieved either a partial response or complete response to their most recent platinum-based chemotherapy.
TESARO is planning to launch the drug in Germany and UK by the end of this year and in other European countries next year.
PARP inhibitors have bright prospects, given their tremendous demand. However, Zejula will face competition from other approved PARP inhibitors, which include AstraZeneca (NYSE:AZN) / Merck’s (NYSE:MRK) Lynparza and Clovis Oncology, Inc.’s (NASDAQ:CLVS) Rubraca. However, Rubraca is under review in Europe.
TESARO claims that Zejula is the most frequently prescribed PARP inhibitor for treating ovarian cancer since its launch in the United States. The sales numbers show better performance by Zejula,
Zejula has generated sales of more than $65 million in the first six months of its launch in the United States, whereas Lynparza’s U.S. sales were $60 million in that period. Also, Rubraca’s sales were $31.5 million.
Similar adoption of the drug in Europe will aid the company.
Approximately 45,000 women are diagnosed with ovarian cancer each year in Europe and more than 22,000 women are diagnosed annually in Europe. Ovarian cancer is the sixth-most common form of cancer and the fifth-most frequent cause of cancer death.
However, Lynparza’s recent approval in the second-line setting can hurt Zejula’s sales.
TESARO carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Hidden Trades
While we share many recommendations and ideas with the public, certain moves are hidden from everyone but selected members of our portfolio services. Would you like to peek behind the curtain today and view them?
Starting now, for the next month, I invite you to follow all Zacks' private buys and sells in real time from value to momentum...from stocks under $10 to ETF to option movers...from insider trades to companies that are about to report positive earnings surprises (we've called them with 80%+ accuracy). You can even look inside portfolios so exclusive that they are normally closed to new investors.
Click here for Zacks' secret trade>>
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
TESARO, Inc. (TSRO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Walgreens Boots Alliance Inc. (NASDAQ:WBA) is on the brink of a significant transformation as it nears a deal with Sycamore Partners to become a private entity. The transaction,...

Using the Elliott Wave Principle (EWP), we have been successfully tracking the most likely path forward for the S&P 500 (SPX) over several months. Although there are many ways...

When looking for dividend stocks, high dividend yields are one important factor to consider. Even if a company’s dividend yield isn’t nearing double-digit percentages, finding...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.