
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Amgen's Osteoporosis Drug Evenity Gets Adverse CHMP View

Amgen Inc. (NASDAQ:AMGN) and Belgian partner UCB announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has rendered a negative opinion on the marketing approval of its osteoporosis drug, Evenity (romosozumab).
Amgen is seeking an approval of Evenity for the treatment of severe osteoporosis in postmenopausal women, who stand at a risk of fracture.
The companies are planning to submit a written notice to the regulatory agency, requesting a re-evaluation of Evenity for the given indication.
Notably, Evenity gained an approval in the United States earlier this April for the treatment of osteoporosis in postmenopausal women, who are at high risk of fracture. However, the nod came with a boxed warning. The drug’s label states that treatment with Evenity may increase the risk level of myocardial infarction (heart attack), stroke and cardiovascular death and that it should not be administered to patients, who already suffered heart attack or stroke in the preceding year. The company is required to conduct a post-marketing study to evaluate the cardiovascular safety of Evenity in postmenopausal osteoporosis women.
The drug had received an approval for a similar indication in postmenopausal women as well as men in Japan, earlier this January.
This CHMP’s response was based on data from three pivotal phase III studies, namely FRAME, ARCH and BRIDGE, which evaluated Evenity in postmenopausal women with osteoporosis, who are at a high risk of fracture and men with osteoporosis.
Evenity treats osteoporosis by increasing bone formation and reducing bone resorption simultaneously. This increases bone mineral density (BMD) and lowers the risk of fracture.
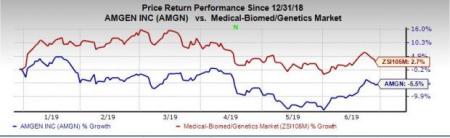
Shares of Amgen have decreased 5.5% so far this year versus the industry’s increase of 2.7%.
We remind investors that Evenity was issued a complete response letter (CRL) by the FDA in July 2017 due to a cardiovascular side effect observed in a study. Last July, Amgen and UCB re-submitted the biologics license application (BLA) to the regulatory body for Evenity.
Notably, Evenity is likely to face stiff competition from Radius Health’s (NASDAQ:RDUS) Tymlos injection, which is already approved for treating postmenopausal women with osteoporosis, who are at a high risk of fracture. Novartis’ (NYSE:NVS) Reclast is also approved for treating osteoporosis.
Zacks Rank & Key Pick
Amgen currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the healthcare sector is Acorda Therapeutics, Inc. (NASDAQ:ACOR) , which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Acorda’s loss per share estimates have been narrowed 6.5% for 2019 and 6.9% for 2020 over the past 60 days.
Will you retire a millionaire?
One out of every six people retires a multimillionaire. Get smart tips you can do today to become one of them in a new Special Report, “7 Things You Can Do Now to Retire a Multimillionaire.”
Novartis AG (NVS): Free Stock Analysis Report
Acorda Therapeutics, Inc. (ACOR): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Radius Health, Inc. (RDUS): Free Stock Analysis Report
Original post
Related Articles

Home improvement retailers Lowe’s (NYSE:LOW) and Home Depot (NYSE:HD) turned a corner, and their Q4 2024 earnings reports confirmed it. The corner is a return to comparable store...

One of our old flames, a former Contrarian Income Portfolio holding, has pulled back sharply in recent weeks. Time to buy the dip in this 4.3% dividend? Let’s discuss. Kinder...

Emini S&P March collapsed on Thursday from strong resistance at 6010/6015The low and high for the last session were 5873 - 6014.(To compare the spread to the contract you...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.