
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Roche's (RHHBY) Tecentriq Gets FDA Nod for Another Indication (Revised)

Roche RHHBY (OTC:RHHBY) recently announced that the FDA has approved immuno-oncology drug Tecentriq (atezolizumab) for another indication.
Tecentriq is now approved as adjuvant treatment, following surgery and platinum-based chemotherapy, for adults with Stage II-IIIA non-small cell lung cancer (NSCLC) whose tumors express PD-L1≥1%, as determined by an FDA-approved test.
The approval was based on results from an interim analysis of the phase III IMpower010 study. Data from the study showed treatment with Tecentriq, following surgery and platinum-based chemotherapy, reduced the risk of disease recurrence or death by 34% in patients with Stage II-IIIA NSCLC (UICC/AJCC 7th edition) whose tumors express PD-L1≥1% compared with best supportive care.
Data from the study has also been submitted as the basis of marketing applications to the European Medicines Agency (“EMA”) and other global health authorities. The review of this application was conducted under the FDA’s Project Orbis initiative, which provides a framework for concurrent submission and review of oncology medicines among international partners.
Approval in additional indications will boost sales of the drug that came in at CHF 1.6 billion in the first half of 2021.
Tecentriq is already approved for various types of lung cancer. It is approved as the front-line treatment of adults with extensive-stage small cell lung cancer (SCLC) in combination with carboplatin and etoposide (chemotherapy). Tecentriq also has four approved indications in advanced NSCLC as either a single agent or in combination with targeted therapies and/or chemotherapies.
Shares of Roche have gained 11.6% so far this year compared with the industry’s growth of 9%.
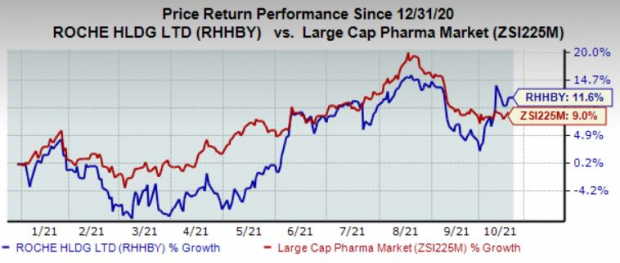
Roche is also evaluating Tecentriq in multiple ongoing and planned phase III studies across different settings in the lung, genitourinary, skin, breast, gastrointestinal, gynecological, and head and neck cancers. Tecentriq is being evaluated both as a standalone treatment and in combination with other medicines, as well as studies in metastatic, adjuvant, and neoadjuvant settings across various tumor types.
In addition to Tecentriq, Roche’s cancer immunotherapy pipeline includes other checkpoint inhibitors, such as tiragolumab, a novel cancer immunotherapy designed to bind to TIGIT, individualized neoantigen therapies and T-cell bispecific antibodies.
While the uptake of Tecentriq has been strong, it is currently facing stiff competition from immuno-oncology therapies like Merck’s MRK Keytruda and Bristol-Myers’ BMY Opdivo in various indications. AstraZeneca (NASDAQ:AZN)’s AZN Imfinzi is also approved for some of these indications.
Roche currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
(We are reissuing this article to correct a mistake. The original article, issued on October 18, 2021, should no longer be relied upon.)
Infrastructure Stock Boom to Sweep America
A massive push to rebuild the crumbling U.S. infrastructure will soon be underway. It’s bipartisan, urgent, and inevitable. Trillions will be spent. Fortunes will be made.
The only question is “Will you get into the right stocks early when their growth potential is greatest?”
Zacks has released a Special Report to help you do just that, and today it’s free. Discover 7 special companies that look to gain the most from construction and repair to roads, bridges, and buildings, plus cargo hauling and energy transformation on an almost unimaginable scale.
Download FREE: How to Profit from Trillions on Spending for Infrastructure >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN): Free Stock Analysis Report
Roche Holding AG (OTC:RHHVF) (RHHBY): Free Stock Analysis Report
Bristol Myers Squibb Company (NYSE:BMY): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
Related Articles

• Trump’s trade war, inflation data, and last batch of earnings will be in focus this week. • DoorDash’s imminent inclusion in the S&P 500 is likely to trigger a wave of...

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.