
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Roche Reports Tecentriq/Avastin Lung Cancer Study Data

Roche’s (OTC:RHHBY) member Genentech, announced interim results from a phase III study evaluating Tecentriq and Avastin plus chemotherapy for first-line treatment of advanced non-squamous non-small cell lung cancer (NSCLC).
The III IMpower150 study showed that Tecentriq and Avastin plus chemotherapy reduced the risk of their disease worsening or death by 38% compared with those who were treated with Avastin plus chemotherapy. Earlier in November, Roche had already mentioned that the III IMpower150 study had met its co-primary endpoint of progression-free survival (PFS).
Concurrent with the present release, the company also announced that the 12-month landmark PFS rate — the percentage of lung cancer patients who survived in a year without their disease progressing — was almost double (37%) in the Tecentriq, Avastin plus chemotherapy arm compared with the Avastin plus chemotherapy arm where the same was 18%.
The secondary endpoint of the study was overall response rate (ORR) — the rate of tumor shrinkage. For the combination of Tecentriq, Avastin plus chemotherapy, tumor shrinkage rate was 64% compared with 48% in the Avastin plus chemotherapy arm.
The Tecentriq study is the first phase III combination trial of a cancer immunotherapy to show improvement in progression-free survival for the first-line treatment of advanced lung cancer.
The company expects to submit these encouraging results to the regulatory authorities for a potential label expansion of Tecentriq. Also, Roche expects to present results from the co-primary endpoint of overall survival (OS) in the first half of 2018.
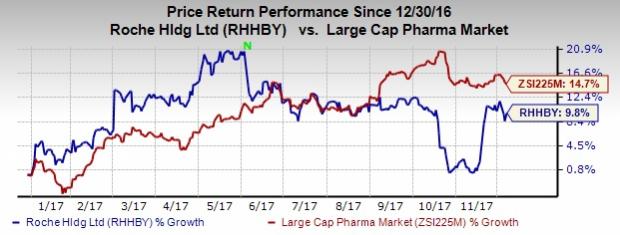
So far this year, shares of the company have increased 9.8% compared with the industry’s gain of 14.7%.
In a separate release, Roche also announced positive interim results from the phase III HAVEN 4 study evaluating Hemlibra (emicizumab) prophylaxis dosed once every four weeks in adults and adolescents (12 years of age or older) with haemophilia A with and without inhibitors to factor VIII.
The study showed that Hemlibra prophylaxis administered by injection under the skin once every four weeks showed meaningful bleed control in people with haemophilia A compared with the current treatment regimens that require more frequent intravenous infusions.
Zacks Rank & Stocks to Consider
Roche has a Zacks Rank #4 (Sell). Some better-ranked health care stocks in the same space are Sucampo Pharmaceuticals (NASDAQ:SCMP) , Johnson and Johnson (NYSE:JNJ) and Corcept Therapeutics Inc. (NASDAQ:CORT) . All of them hold a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Sucampo’s earnings per share estimates have moved up from 3 cents to $1.12 for 2017 and from $1.15 to $1.19 for 2018, over the last 30 days. The company delivered a positive earnings surprise in three of the trailing four quarters, with an average beat of 15.63%. The share price of the company has increased 10.3% year to date.
Johnson and Johnson’s earnings per share estimates have moved up from $7.18 to $7.28 for 2017 and from $7.72 to $7.85 for 2018, over the last 60 days. The company came up with a positive earnings surprise in all of the trailing four quarters, with an average beat of 3.12%. The share price of the company has increased 22.4% year to date.
Corcept’searnings per share estimates have moved up from 45 cents to 47 cents for 2017 and from 77 cents to 88 cents for 2018, over the last 60 days. The company pulled off a positive earnings surprise in two of the trailing four quarters, with an average beat of 14.32%. The share price of the company has increased 123.3% year to date.
Zacks' Hidden Trades
While we share many recommendations and ideas with the public, certain moves are hidden from everyone but selected members of our portfolio services. Would you like to peek behind the curtain today and view them?
Starting now, for the next month, I invite you to follow all Zacks' private buys and sells in real time from value to momentum...from stocks under $10 to ETF to option movers...from insider trades to companies that are about to report positive earnings surprises (we've called them with 80%+ accuracy). You can even look inside portfolios so exclusive that they are normally closed to new investors.
Click here for Zacks' secret trade>>
Roche Holding AG (RHHBY): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Sucampo Pharmaceuticals, Inc. (SCMP): Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

In a market fraught with uncertainty, investors often seek refuge in defensive-minded stocks that offer stability and resilience. Two such stalwarts, Johnson & Johnson and...

Bitcoin has gained legitimacy as it has achieved mainstream status these days. Even the United States Securities and Exchange Commission (SEC) has acknowledged its legitimacy with...

Stocks fell sharply, with the S&P 500 leading the decline, finishing the day down almost 1.6% at 5,860. Meanwhile, the Nasdaq 100 dropped nearly 2.75%, closing at 20,550. This...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review


Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.