
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

AbbVie (ABBV) Stock Hits 52-Week High: More Room To Run?

AbbVie Inc. (NYSE:ABBV) shares hit a 52-week high of $71.12 on Friday before eventually closing at $71.05. In fact, shares of the company are up 13.4% since the beginning of this year, outperforming the Zacks classified Large Cap Pharmaceuticals industry, which increased 11.4% in the same period.
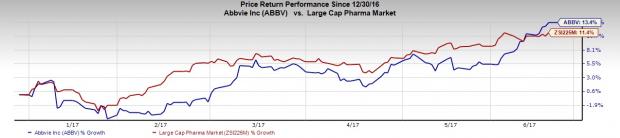
Strong first-quarter 2017 results coupled with positive pipeline data read-outs and regulatory updates this year fueled the upside. Let’s analyze the factors that will help the company to continue on this trajectory.
Factors at Play
The company’s flagship rheumatoid arthritis drug Humira accounted for almost 63% of total revenue in the first quarter of 2017, registering growth of nearly 15% year over year. The drug is the main driver of AbbVie’s revenues, currently approved for several indications. The company is also conducting studies to expand its label into other indications. The company expects Humira to bring in total sales of more than $18 billion in 2020.
However, quite a few companies are working on bringing Humira biosimilars to the market.
A biosimilar from Amgen Inc. (NASDAQ:AMGN) was approved by the FDA in 2016. However, the drug has not yet been launched due to ongoing litigation. In May 2017, AbbVie lost a lawsuit to Coherus Biosciences, Inc. (NASDAQ:CHRS) regarding the dosing patent ‘135 for Humira. However, shares weren’t affected much as the investment community believes that AbbVie has a robust intellectual property (IP) portfolio beyond the ‘135 patent, which should preclude biosimilars from the U.S. market until 2022. Sales should continue to remain strong until then.
Although Humira generates most of its revenues, the company has diversified its revenue stream with the addition of Imbruvica to its portfolio. Imbruvica sales increased 44.6% from the year-ago quarter to $551 million in the first quarter of 2017. The company expects Imbruvica peak sales of more than $7 billion and revenues of about $5 billion in 2020. Imbruvica, currently approved for quite a few indications, has multi-billion dollar potential. AbbVie intends to expand Imbruvica’s label into solid tumors and autoimmune diseases. AbbVie co-owns the drug with Johnson & Johnson (NYSE:JNJ) .
With expected growth in Humira and Imbruvica sales over the next few years, the company’s top line should see an upside. Moreover, AbbVie also has a strong pipeline with several late-stage candidates. The company is developing risankizumab for psoriasis and Crohn's disease while ABT-494 is being developed for treating rheumatoid arthritis (RA).
In the last couple of months, AbbVie announced positive top-line results from a phase III study on upadacitinib (ABT-494) for RAas well as a phase II study for Crohn's disease. These played an important role in driving up the share price. Meanwhile, AbbVie’s RBV-free once-daily pan-genotypic combinationregimen, glecaprevir/pibrentasvir (G/P),is under accelerated assessment in the EU and priority review in the U.S. and Japan with commercialization expected this year.Also, the company announced some label updates for both Imbruvica and Humira.
Venetoclax, an oncology pipeline candidate was approved by the FDA in 2016 for chronic lymphocytic leukemia (CLL) and is being studied in other indications as well.
Investors are focused on the performance of AbbVie’s HCV portfolio as the segment is facing competition from Gilead’s Sovaldi and Harvoni along with increasing pricing pressure. Viekira suffered a year-over-year drop of 36.5% in its sales in the first quarter of 2017 to $263 million. The weak performance is expected to continue this year with sales expected to decline to $1 billion in 2017 from $1.5 billion in 2016.
Despite these pressures, AbbVie looks well poised to deliver strong earnings and sales growth through the rest of this year backed by its key drugs Imbruvica and Humira. Meanwhile, investor focus will remain on the stock with several late-stage data-readouts expected in the second half as well.
Attractive Numbers
The stock trades at an attractive valuation as evident from its favorable P/E F12M (forward twelve months), which stands at 11.84 and trades at a discount to large cap pharmaceuticals industry’s P/E F12M of 15.91. Moreover, earnings are expected to increase 14.62% in 2017 and 18.59% in 2018. The numbers indicate that the stock has the potential to rise further.
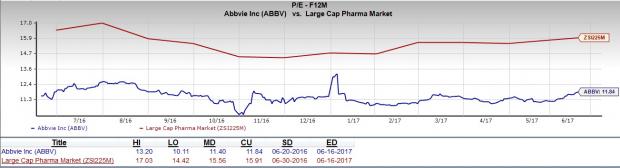
Moreover, the company has delivered a positive surprise in two of the trailing four quarters and met estimates in the remaining two quarters, with an average four-quarter surprise of 1.65%.
Zacks Rank
AbbVie currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look. See the pot trades we're targeting>>
Johnson & Johnson (JNJ): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Coherus BioSciences, Inc. (CHRS): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Home improvement retailers Lowe’s (NYSE:LOW) and Home Depot (NYSE:HD) turned a corner, and their Q4 2024 earnings reports confirmed it. The corner is a return to comparable store...

One of our old flames, a former Contrarian Income Portfolio holding, has pulled back sharply in recent weeks. Time to buy the dip in this 4.3% dividend? Let’s discuss. Kinder...

Emini S&P March collapsed on Thursday from strong resistance at 6010/6015The low and high for the last session were 5873 - 6014.(To compare the spread to the contract you...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.