
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Radius (RDUS) Q4 Loss Wider Than Expected On Higher Expenses

Radius Health, Inc. (NASDAQ:RDUS) reported a loss of $1.59 per share in the fourth quarter of 2017, wider than the Zacks Consensus Estimate loss of $1.48 and the year-ago quarter loss of $1.22. Increase in general and administrative expenses led to the wider than anticipated net loss year over year.
The company reported sales of Tymlos (abaloparatide) of $7.7 million surpassing the Zacks Consensus Estimate of $6.7 million.
Quarter in Detail
Research and development expenses for the reported quarter were $22.9 million, down 10.5% year over year due to the reduction in R&D expenses associated with the elacestrant projects and the development of Tymlos. Radius Health received FDA approval for Tymlos in April 2017 for the treatment of postmenopausal women with osteoporosis at high risk of fracture and began shipments to wholesalers at the end of May 2017.
Tymlos continues to gain traction with approximately 259 million covered lives and 93% coverage in commercial plans. Tymlos market share reached approximately 32% of new patients starting anabolic therapy (NBRx) and 13% of total prescriptions in the anabolic market.
General and administrative expenses for the reported quarter jumped to $50.7 million from $27.5 million primarily due to personnel, promotional, and consulting expenses related to the launch of Tymlos.
Pipeline Updates
The company is developing two formulations of abaloparatide-SC and abaloparatide-transdermal. A labeling supplement for Tymlos was submitted to the FDA in the fourth quarter with the positive 43-month data from the ACTIVExtend study, which showed continued significant risk reduction in fractures in patients who were transitioned to receive an additional 24 months of bisphosphonate therapy. In January 2018, Radius Health met with the FDA to discuss the regulatory development path for the abaloparatide patch. The FDA agreed that, depending on the study results, a single, randomized, open label, active-controlled, non-inferiority (to abaloparatide-SC) study of up to 500 patients with postmenopausal osteoporosis at high risk of fracture would be sufficient to gain approval for the abaloparatide patch. The primary endpoint in the study will be change in lumbar spine bone mineral density at 12 months. Radius Health expects to initiate this pivotal trial in mid-2019.
Meanwhile, Radius Health’ Marketing Authorisation Application “(MAA”) for Eladynos (abaloparatide-SC) for the treatment of postmenopausal women with osteoporosis in Europe is under review by the Committee for Medicinal Products for Human Use (“CHMP”) of the European Medicines Agency (“EMA”). The company however suffered a setback when the CHMP issued a second Day-180 List of Outstanding Issues. In December 2017, the CHMP issued a third Day-180 List of Outstanding Issues and informed the company that it intends to refer the MAA to a scientific advisory group for additional advice. The Company expects an opinion from the CHMP regarding the MAA in the first half of 2018. Radius Health and the FDA have also agreed on the design of a clinical trial in men with osteoporosis, which, if successful, will form the basis of an sNDA seeking to expand the use of Tymlos to treat men with osteoporosis at high risk for fracture. The study is expected to be initiated in the first quarter of 2018.
The company also plans to initiate a clinical trial in men with osteoporosis, which, if successful, will form the basis of an sNDA seeking to expand the label to treat men with osteoporosis at high risk for fracture. The study is expected to be initiated in the first quarter of 2018.
Meanwhile, the company presented positive data on elacestrant for breast cancer in December 2017. The FDA also granted Fast Track designation to elacestrant in October 2017.
In February 2018, Radius Health obtained scientific advice from the EMA regarding a potential single-arm monotherapy phase II trial of elacestrant in patients with ER-positive/HER2-negative advanced or metastatic breast cancer. Based on feedback from the EMA and the FDA, the company will conduct a single, randomized, controlled phase II trial of elacestrant as a third-line monotherapy in approximately 300 patients with ER positive/HER2 negative advanced/metastatic breast cancer.
Our Take
The company’s wider-than-expected loss in the fourth quarter was due to escalated expenses with the commercialization of Tymlos. The drug continues to gain traction and grab further market share.
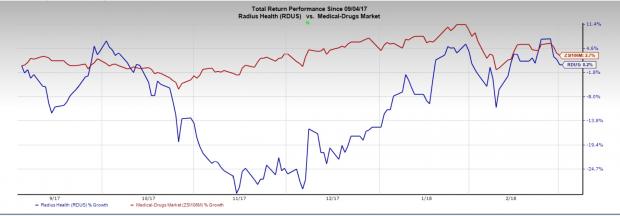
Radius Health’ shares have gained 0.2% in the last six months compared with the industry’s gain of 2.7%.
Although the osteoporosis market in the United Sates has great potential as approximately 1.4 million postmenopausal women experience an osteoporotic fractur each year, Tymlos is expected to face significant competition from Eli Lilly &Co's (NYSE:LLY) Forteo and Amgen's (NASDAQ:AMGN) Prolia.
Moreover, the approved label carries a boxed warning of osteosarcoma (a malignant bone tumor). The company also a setback when the CHMP issued a third Day-180 List of Outstanding Issues.
Zacks Rank & Key Pick
Radius Health currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the health care sector is Regeneron Pharmaceuticals (NASDAQ:REGN) which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Regeneron’s earnings per share estimates have moved up from $17.13 to $18.65 and from $20.38 to $21.56 for 2018 and 2019, respectively, in the last 30 days. The company pulled off a positive earnings surprise in three of the last four quarters with an average beat of 9.15%.
Don’t Even Think About Buying Bitcoin Until You Read This
The most popular cryptocurrency skyrocketed last year, giving some investors the chance to bank 20X returns or even more. Those gains, however, came with serious volatility and risk. Bitcoin sank 25% or more 3 times in 2017.
Zacks’ has just released a new Special Report to help readers capitalize on the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly less volatility than buying them directly.
See 4 crypto-related stocks now >>
Eli Lilly and Company (LLY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Radius Health, Inc. (RDUS): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.