
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

QIAGEN Releases Coronavirus Test Kit In US On New FDA Policy

QIAGEN N.V. (NYSE:QGEN) announced that it started shipping its new QIAstat-Dx Respiratory SARS-CoV-2 Panel test (which is to be sold as an in-vitro diagnostic or IVD) in the United States to help diagnose coronavirus-infected patients. The announcement came after a new policy of an Emergency Use Authorization (EUA) was declared by the FDA early this month. Notably, the company will submit the related EUA to the FDA this week.
Per QIAGEN, QIAstat-Dx SARS-CoV-2 test kits currently available in the United States are the first syndromic tests, which can detect SARS-CoV-2 as well as more than 20 other respiratory targets. The company is also on track to ramp up its test kit production to help contain the coronavirus outbreak.
Additionally, QIAGEN is currently supplying RNA extraction kits to the United States and other countries to accelerate the efforts to contain the outbreak.
With the efforts, QIAGEN aims to strengthen its foothold in the global Molecular Diagnostics market.
Background Prior to the Release of the Test Kits
The release of the test kits was preceded by the announcement of QIAGEN receiving funding for the project in part from the U.S. Department of Health and Human Services' Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority Division of Research, Innovation, and Ventures.
Significance of the Release
The QIAstat-Dx SARS-CoV-2 test kits, which QIAGEN has already started shipping to other countries from February for clinical validation, runs on the company’s Sample to Insight syndromic system. The syndromic system automates molecular analysis to deliver differential diagnosis in about an hour. The automation system enables fast, cost-effective and easy-to-use syndromic testing with Sample to Insight workflows, thus speeding up the result delivery.
Additionally, the panel detects a few other pathogens and subtypes of respiratory infections like Adenovirus, Coronavirus 229E, Coronavirus HKU1, Coronavirus NL63 and Coronavirus OC43. However, given the current increasing testing demand, the regulatory status of the QIAstat-Dx Respiratory SARS-CoV-2 Panel will vary by location.
Industry Prospects
Per a report by Grand View Research, the global IVD market size was valued at $60.8 billion in 2019, and is expected to witness a CAGR of 4.4% between 2020 and 2027. Factors like growth in the prevalence of chronic and infectious diseases, increasing adoption of fully automated instruments, and automation in laboratories are expected to drive the market.
Given the global condition of the market amid the COVID-19 outbreak, QIAGEN seems to have huge market potential.
Recent Developments in Molecular Diagnostics
Of late, QIAGEN has been working toward combatting the COVID-19 outbreak.
The company is currently ramping up production of test kits for the detection of SARS-CoV-2 in patients. In March, it received the CE mark for its QIAstat-Dx Respiratory SARS-CoV-2 Panel test for the detection of SARS-CoV-2. The product is now available for use by the clinical laboratories in Europe. Further, QIAGEN obtained derogation in Germany by the Bundesinstitut für Arzneimittel und Medizinprodukte for the test.
In February, the company announced that it is currently providing various testing solutions for SARS-CoV-2 like enabling laboratory-developed tests, mid- and high-throughput automation, and two new real-time polymerase chain reaction (“RT-PCR”) tests for the detection of SARS-CoV-2. However, the RT-PCR tests (developed at QIAGEN sites in China and the United States) will be available for research purposes only.
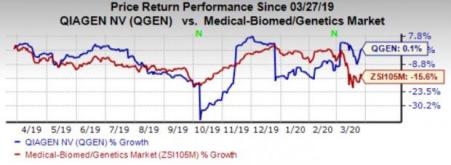
Price Performance
Shares of QIAGEN have gained 0.1% in the past year against the industry’s 15.7% decline.
Zacks Rank & Other Key Picks
Currently, the company carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks from the broader medical space are ResMed Inc. (NYSE:RMD) , Medtronic plc (NYSE:MDT) and Hill-Rom Holdings, Inc. (NYSE:HRC) .
ResMed has a projected long-term earnings growth rate of 14.4%. It currently carries a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Medtronic’s long-term earnings growth rate is estimated at 7.1%. The company presently has a Zacks Rank #2.
Hill-Rom’s long-term earnings growth rate is estimated at 11.1%. It currently carries a Zacks Rank #2.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +24.5% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Medtronic PLC (MDT): Free Stock Analysis Report
ResMed Inc. (RMD): Free Stock Analysis Report
QIAGEN N.V. (QGEN): Free Stock Analysis Report
Hill-Rom Holdings, Inc. (HRC): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.