
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Pfizer Plans Coronavirus Therapy, Progresses With Tanezumab BLA

Pfizer Inc. (NYSE:PFE) announced that it has identified certain antiviral compounds, which were already in development, with potential to treat coronavirus-affected people. The company is currently engaged in screening the compounds. It is planning to initiate clinical studies on these compounds by year-end, following any positive results, expected by this month end.
In the wake of rising coronavirus infection in the United States and across the globe, several large and small pharma/biotech are engaged in developing a vaccine or a treatment. Globally, Covid-19 has already infected more than 90,000 people and the death toll has crossed the 3000-mark. Coronavirus has infected more than 90 people in the United States and caused six deaths. The World Health Organization (“WHO”) declared Covid-19 as a global emergency on Jan 30. The WHO and other global organizations and government authorities are actively participating in developing treatment for Covid-19 and spreading awareness to avoid infection.
The U.S. federal authorities met several pharma/biotech companies on Monday to discuss the progress of any treatment for Covid-19. The Trump administration urged companies to collaborate for accelerated development of a vaccine. Representatives from various companies at the meeting suggested 12 to 18 month timeline for a safe and tested vaccine to be available in the market. However, antiviral treatments, which are already being developed for other viral infections, are expected to get faster approval and may lead to earlier availability of Covid-19 treatment.
Currently, AbbVie’s (NYSE:ABBV) HIV drug, Kaletra, is being administered to coronavirus-affected patients in China. Gilead (NASDAQ:GILD) initiated two phase III studies last month to evaluate its antiviral candidate, remdesivir, as a treatment for Covid-19. Pipeline candidates of other companies are in earlier stages of development.
In the past year, shares of Pfizer have declined 19% compared with the industry’s decrease of 2.5%.
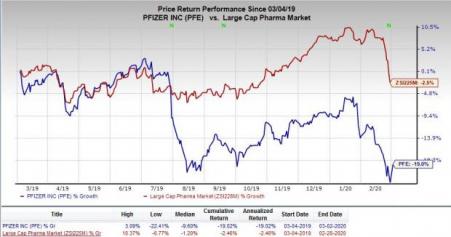
Meanwhile, Pfizer issued a press release on Mar 2 announcing the FDA acceptance of a biologics license application ("BLA") seeking approval for subcutaneous administration of tanezumab in patients with chronic pain due to moderate-to-severe osteoarthritis (“OA”). A decision is expected in December 2020. The company has collaboration with Eli Lilly (NYSE:LLY) for the development and commercialization of the candidate.
The BLA included data from 39 clinical studies, including three late-stage studies, which evaluated tanezumab in patients with moderate-to-severe OA. Data from these studies have shown that treatment with tanezumab led to statistically significant improvement in pain, physical function and the respective patients’ overall assessment of their OA.
Zacks Rank
Pfizer currently carries a Zacks Rank #2 (Buy).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +50%, +83% and +164% in as little as 2 months. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
Pfizer Inc. (PFE): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Original post
Related Articles

Through many years of frustration among gold bugs due to the failure of gold stock prices to leverage the gold prices in a positive way, there were very clear reasons for that...

I know there is the smell of fear in the air when I see my readership double as we reach a point where weekly chart factors come into play. Up until last week, markets have...

Professional traders get paid because of one skill and one skill only: the ability to foresee what the world (or the economy, at least) might look like in six to nine months....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.