
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Novavax Rallies As Influenza Vaccine Meets All Goals In Study

Shares of Novavax, Inc. (NASDAQ:NVAX) gained 18.7% yesterday after the company announced positive top-line data from a pivotal phase III study on its nanoparticle seasonal influenza vaccine candidate, NanoFlu, for adult patients aged 65 years and above.
In fact, the stock has rallied 20.3% in the past year against the industry’s decline of 19.9%.
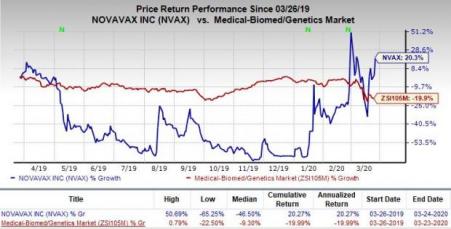
With help of the FDA’s criteria for accelerated approval of seasonal influenza vaccines, the phase III study on NanoFlu evaluated the safety and immunogenicity of the candidate using Novavax's proprietary Matrix-M adjuvant in senior patients aged 65 years and older compared to the quadrivalent formulation of Sanofi’s (NASDAQ:SNY) influenza vaccine, Fluzone.
The primary objectives of the study were to demonstrate non-inferior immunogenicity of NanoFlu as measured by hemagglutination inhibition (HAI) titers of vaccine homologous influenza strains compared to Fluzone High-Dose and also show NanoFLu’s safety profile.
The study met all the primary goals as well as achieved statistical significance in key secondary endpoints. Novavax plans to submit a biologics license application (BLA) for NanoFLu using the FDA’s accelerated approval pathway. The company expects to make NanoFLu available in the market as early as possible.
Notably, in January 2020, the FDA granted a Fast Track designation to NanoFlu for adult patients aged 65 years and above. In June 2019, the regulatory agency had acknowledged Novavax’s plans to use the accelerated approval pathway for NanoFlu.
We remind investors that recently, Novavax was in the spotlight following its launched efforts to develop a novel vaccine to protect from the coronavirus disease COVID-19.
In March, the Coalition for Epidemic Preparedness (CEPI) awarded an initial funding of $4 million to support Novavax’s efforts to develop a COVID-19 vaccine. Both CEPI and the company are in talks regarding additional funding from CEPI to cover the costs through the phase I.
CEPI also provided funding to other small-scale biotechs like Moderna, Inc. (NASDAQ:MRNA) , CureVac and Inovio Pharmaceuticals, Inc. (NASDAQ:INO) to push potential vaccine candidates for early-stage clinical studies within a few months.
The World Health Organization declared the COVID-19 outbreak a pandemic, given the alarming levels of its spread and severity. With the global coronavirus outbreak relentlessly posing a threat to human health, a speedy development of vaccines is the need of the hour.
We remain optimistic about the developments in pharma sector as several companies along with global authorities are laboring hard on rolling out a treatment as early as possible to neutralize this deadly virus.
Zacks Rank
Novavax currently carries Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Sanofi (PA:SASY) (SNY): Free Stock Analysis Report
Moderna, Inc. (MRNA): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.