
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Novartis Reports Updated Results From Kymriah's JULIET Study

Novartis AG (NYSE:NVS) announced updated results from the pivotal JULIET study. It showed sustained complete responses at six months with Kymriah suspension for intravenous infusion, in adult patients with a difficult-to-treat cancer- relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL).
Kymriah (tisagenlecleucel), formerly CTL019, is the first chimeric antigen receptor T cell (CAR-T) therapy approved. The drug has been developed in collaboration with the University of Pennsylvania. This novel immunocellular therapy and one-time treatment uses patient's T cells to fight cancer.
CAR T therapies are developed for individual patients using their own cells, thereby making it different from typical small molecule or biologic therapies.
So far this year, Novartis’ shares have gained 15.2%, compared with the industry’s growth of 15.6%.
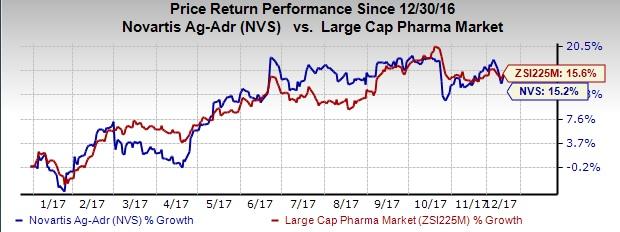
JULIET is the first multi-center global registration study for Kymriah in adult patients with r/r DLBCL and the second global CAR-T cell therapy trial. In the study at six months, 30% of patients treated with Kymriah showed complete response. The relapse-free probability at six months after first response was 74%. Median overall survival was not achieved and the median time from infusion to data cutoff was 5.6 months.
When the patients with DLBCL enrolled for this study, they had to go through multiple rounds of chemotherapy and many had unsuccessful stem cell transplants, leaving them with few options and a poor prognosis. The study showed that Kymriah was able to considerably increase their chance of achieving and maintaining a sustained response without stem cell transplant, thus highlighting its benefit.
Additionally, the study revealed that cytokine release syndrome (CRS) occurred in 58% of the total patients under treatment while 23% experienced grade 3/4 CRS (15% grade 3; 8% grade 4) using the Penn Grading Scale. Also, it showed that 21% of patients experienced any grade neurologic events and 12% of patients had grade 3/4 neurologic adverse events, who were provided supportive care.
In the study, 26 patients (26%) were infused in the outpatient setting of which 20 patients (77%) remained outpatient for three or more days after infusion.43 patients discontinued before infusiondue to rapid progression of their disease or deterioration in their clinical status.
According to the study, only nine out of 147 (6.1%) enrolled patients could not be infused due to inability to manufacture an adequate dose of CAR-T cells.
Novartis submitted a supplemental Biologics License Application (sBLA) to the FDA in October for Kymriah suspension for intravenous infusion. The applicationwas filed for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) who are ineligible for autologous stem cell transplant (ASCT).
Again in November, the company submitted an application to the European Medicines Agency (EMA) for Kymriah for the treatment of adult patients with r/r DLBCL who are ineligible for ASCT and for children as well as young adults with r/r B-cell ALL.
Of late, Gilead (NASDAQ:GILD) also received FDA approval for Yescarta — a CAR-T therapy for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy. Yescarta is the first CAR-T therapy approved by the FDA for this indication.
Another company, bluebird bio, Inc.’s (NASDAQ:BLUE) anti-BCMA CAR T therapy, bb21217, is being evaluated for relapsed/refractory multiple myeloma. bb21217 as well as bb2121 are being developed in collaboration with Celgene (NASDAQ:CELG) .
Zacks Rank
Novartis carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Stocks from Zacks' Hottest Strategies
It's hard to believe, even for us at Zacks. But while the market gained +18.8% from 2016 - Q1 2017, our top stock-picking screens have returned +157.0%, +128.0%, +97.8%, +94.7%, and +90.2% respectively.
And this outperformance has not just been a recent phenomenon. Over the years it has been remarkably consistent. From 2000 - Q1 2017, the composite yearly average gain for these strategies has beaten the market more than 11X over. Maybe even more remarkable is the fact that we're willing to share their latest stocks with you without cost or obligation.
Novartis AG (NVS): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
bluebird bio, Inc. (BLUE): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Since the Robotaxi event on October 11th, Tesla (NASDAQ:TSLA) stock is up 38%, currently priced at $291.60 per share This is a return to the early November 2024 price level. But...

The Q4 2024 earnings season tapers off from here, with S&P 500® EPS growth surpassing 17%, the highest in 3 years Large cap outlier earnings dates this week include:...

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.