
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Nektar/Bristol-Myers' Combo Trial Positive In Phase I/II

Nektar Therapeutics (NASDAQ:NKTR) along with partner Bristol-Myers Squibb (NYSE:BMY) announced positive interim data from dose-escalation part of a phase I/II study, evaluating the safety and efficacy of the combination of its lead candidate, NKTR-214, with Bristol-Myers’ Opdivo (nivolumab). The combination therapy is being evaluated for treatment of patients with melanoma, renal cell carcinoma and non-small cell lung cancers (NSCLC).
Data from the study was presented at the 2017 annual meeting of Society for Immunotherapy of Cancer (SITC).
Notably in September last year, Nektar had entered into a clinical collaboration agreement with Bristol-Myers for evaluating the Opdivo/NKTR-214 combination therapy across five tumor types (melanoma, kidney, colorectal, bladder and non-small cell lung cancer) and eight potential indications in phase I/II studies. While both companies share cost of the combination studies equally, Nektar retains the global commercial rights to NKTR-214.
Nektar’s shares have significantly outperformed the industry so far this year. The stock has skyrocketed 164.9% while the industry has increased 0.9%.
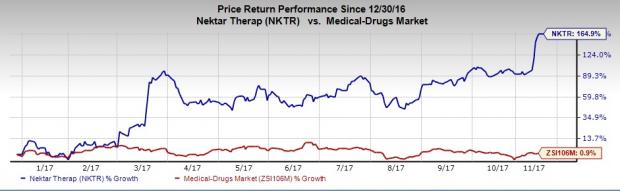
The PIVOT-02 phase I/II study was conducted on a total of 38 patients across a number of dose cohorts. Data from the trial demonstrated positive response rates across all three tumor types in both PD-L1 positive and PD-L1 negative patients.The company reported no treatment discontinuation due to adverse events.
Importantly, NKTR-214 is a CD122-biased agonist, while Opdivo is a PD-1 immune checkpoint inhibitor. The two different and complementary mechanisms, which comprise the combination regimen, could provide new treatment options for cancer patients.
We remind investors that Nektar is also conducting a phase I/II PROPEL study to evaluate the efficacy and safety of NKTR-214 in combination with Roche's (OTC:RHHBY) Tecentriq (atezolizumab) and Merck's (NYSE:MRK) Keytruda (pembrolizumab). The PROPEL study complements Nektar’s ongoing PIVOT trial.
Earlier in May, Nektar had entered into a research collaboration with Takeda Pharmaceuticals to explore the combination of NKTR-214 with five oncology compounds from Takeda’s cancer portfolio.
Zacks Rank
Nektar carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Nektar Therapeutics (NKTR): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

• Trump’s trade war, inflation data, and last batch of earnings will be in focus this week. • DoorDash’s imminent inclusion in the S&P 500 is likely to trigger a wave of...

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.