
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

J&J Presents Positive New Data For Diabetes Drug Invokana

Johnson & Johnson (NYSE:JNJ) presented new data from a large CANVAS outcomes program on its type II diabetes drug, Invokana (SGLT2 inhibitor), demonstrating an improved renal outcome. The data was presented at the annual meeting of the American Heart Association Scientific Sessions in California and published in the Circulation.
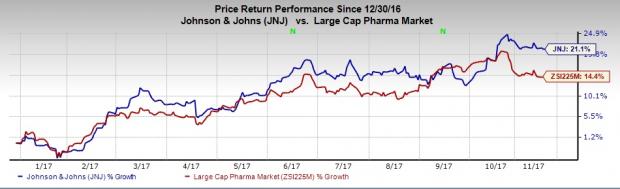
So far this year, Johnson & Johnson’s share price has increased 21.1%, comparing favorably with the 14.4% rally of the industry it belongs to.
This new analysis of CANVAS showed a reduction in the risk of the composite renal endpoint in both patient groups — with a history of CV disease (secondary prevention) and those with only risk factors for CV disease (primary prevention). Also, Invokana demonstrated reduction in the risk of hospitalization due to heart failure (HHF) in both patient groups. No new adverse events were reported during this additional analysis.
We remind investors that in June, the company reported data from the CANVAS program, which demonstrated that Invokana was successful in reducing the risks of cardiovascular (CV) outcomes in type II diabetes patients, who have established cardiovascular (CV) disease or are at a risk for CV disease. Invokana reduced major adverse CV events or MACE by 14% compared with placebo. MACE is a composite endpoint of CV death, non-fatal myocardial infarction or non-fatal stroke. However, the study also showed that the drug increased the risk of amputations.
Last month, J&J submitted a supplemental new drug application (sNDA) to the FDA for the label expansion of Invokana to include the cardiovascular indication based on the data from the CANVAS program.
Many pharma companies are working hard to get the labels of their diabetes medicines updated to include the cardiovascular benefits. With death from cardiovascular diseases being significantly higher in adults with diabetes compared with those without it, the addition of positive CV outcomes on labels of diabetes drugs can give sales a shot in the arm.
Eli Lilly & Company (NYSE:LLY) received both FDA and EU approvals last year to include the CV risk reduction data from the EMPA-REG OUTCOME study on the label of Jardiance. Additionally, in August, Novo Nordisk’s (NYSE:NVO) Victoza (liraglutide) was approved by the FDA for a new indication to reduce the risk of major adverse cardiovascular (CV) events in adults with type II diabetes and established CV disease.
However, in May, Merck & Co., Inc. (NYSE:MRK) was denied an approval by the FDA to include cardiovascular outcomes data from the TECOS trial on the labels of its DPP-IV inhibitor, Januvia (sitagliptin) and other medicines containing Januvia.
AstraZeneca’s Bydureon also failed to reduce the cardiovascular risk in a phase IIIb/IV cardiovascular outcomes study, EXSCEL.
Zacks Rank
Johnson & Johnson carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Eli Lilly and Company (LLY): Free Stock Analysis Report
Novo Nordisk (CO:NOVOb) A/S (NVO): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Defense stocks took a tumble heading into 2025 as President Trump returned to the White House for his second term. Trump has stated his intent as a peacemaker to bring the wars in...

Using the Elliott Wave Principle (EWP), we have been tracking the most likely path forward for the Nasdaq 100 (NDX). Although there are many ways to navigate the markets and to...

Investors are on edge about what tariff policy means for markets Coming off a strong Q4 earnings season, fresh February corporate sales figures can help assess the macro...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.