
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Jazz's (JAZZ) Xyrem Follow-On Candidate NDA Gets FDA Acceptance

Jazz Pharmaceuticals plc (NASDAQ:JAZZ) announced that the FDA has accepted the new drug application (“NDA”) seeking approval for JZP-258 as a treatment for cataplexy and excessive daytime sleepiness (“EDS”) associated with narcolepsy in patients seven years or older. The FDA has also granted priority review to the NDA and a decision is expected by Jul 21, 2020.
JZP-258 is a follow-on candidate of the company’s blockbuster sleep disorder drug, Xyrem. It is an oxybate product candidate with 92% less sodium content than Xyrem, which is also approved for cataplexy or EDS sleepiness associated with narcolepsy.
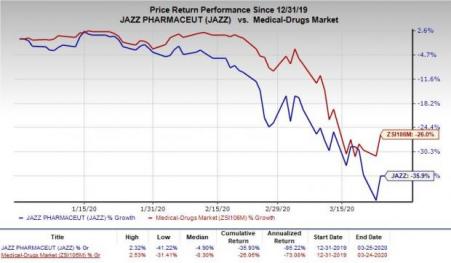
Jazz’s shares have declined 35.9% so far this year compared with the industry’s decrease of 26%.
The NDA for JZP-258 was based on data from a late-stage study, which evaluated the candidate for change in weekly cataplexy attacks compared to a placebo. Study data showed that treatment with JZP-258 achieved highly statistically significant differences in the number of cataplexy attacks as well as in key secondary endpoint of change in Epworth Sleepiness Scale score versus placebo. The candidate demonstrated a safety profile similar to Xyrem.
The company’s strong sleep disorder portfolio has two FDA-approved drugs — Xyrem and Sunosi (solriamfetol). Xyrem, its key revenue generator, recorded full-year sales of $1.64 billion, representing year-over-year growth of 17% and constituting 76% of total revenues in 2019. The company expects total sales from Xyrem and JZP-258, following its potential launch, to be in the range of $1.71-$1.76 billion in 2020.
Meanwhile, Sunosi was launched in the United States for treating EDS in patients with narcolepsy (with or without cataplexy) or obstructive sleep apnea in July 2019. The drug generated nearly $3.7 million in sales in 2019.
Potential approval to JZP-258 will boost Jazz’s sleep disorder portfolio further as JZP-258 is likely to expand the eligible patient population for Jazz’s medicines. It will likely include patients ineligible for Xyrem as they are at risk of high sodium intake-related consequences, including hypertension and other cardiovascular diseases. The highest approved dose of Xyrem of 9 grams per night contains 1,640 mg of sodium, which is almost 70% of the recommended daily sodium intake for a healthy adult per an article published on FDA’s website.
Moreover, Xyrem is set to face generic competition as early as 2023. The low sodium content of JZP-258 and a similar safety profile are likely to largely offset the decline in Xyrem sales once generic versions enter the market.
Approved drugs for narcolepsy include Teva Pharma’s (NYSE:TEVA) Provigil and Novartis’ (NYSE:NVS) Ritalin-SR. A few other pharma companies are also developing treatment for narcolepsy, which includes Avadel Pharmaceuticals (NASDAQ:AVDL) .
Apart from sleep disorder, Jazz also has a few oncology drugs in its portfolio. The company is also expanding its cancer portfolio through internal development as well as signing partnerships.
Zacks Rank
Jazz currently has a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +24.5% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Teva Pharmaceutical Industries Ltd. (TEVA): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Jazz Pharmaceuticals PLC (JAZZ): Free Stock Analysis Report
Avadel Pharmaceuticals PLC. (AVDL): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...

Nvidia is scheduled to release its Q4 earnings report at 4:20PM ET on Wednesday. A call with CEO Jensen Huang is set for 5:00PM ET. The chipmaker’s results will serve as a...

Warren Buffett has always critiqued airline stocks for being overly capital-intensive, exhibiting low growth, and relying heavily on cyclical consumer travel patterns—further...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.