
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Inovio Surges On Expedited Timeline For Coronavirus Vaccine

Inovio Pharmaceuticals, Inc. (NASDAQ:INO) announced that it expects to begin human clinical studies on its DNA vaccine INO-4800 in April for addressing the novel coronavirus (Covid-19). Clinical studies in China and South Korea are expected to begin shortly thereafter.
Shares of Inovio soared 69.7% on Tuesday following the news. In fact, the stock has skyrocketed 106.4% in the past year against the industry’s decline of 7.3%. Moreover, the shares were again up 24% in pre-market trading on Wednesday.
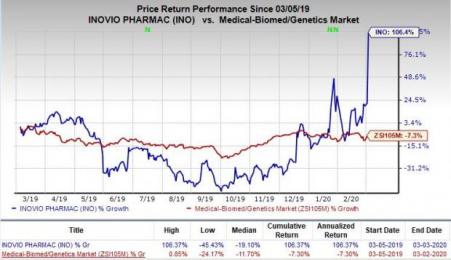
Currently, INO-4800 is in preclinical testing, designed to precisely match the DNA sequence of the deadly virus. In January 2020, Inovio received a grant of up to $9 million from CEPI for the pre-clinical and phase I development of INO-4800.
Importantly, by this year-end, Inovio plans to deliver one million doses of INO-4800 for further clinical studies and emergency use to treat the coronavirus-affected people.
In the wake of this rapidly-spreading coronavirus infection in China and across the globe, several large and small pharma/biotech players are making concerted efforts to develop a vaccine or a treatment. Per the latest Bloomberg article, globally, Covid-19 already infected more than 90,000 people and the death toll crossed the 3000-mark. Coronavirus afflicted more than 100 people in the United States, killing eight of them.
The WHO and other global organizations as well as government authorities are actively participating in developing treatments for the Covid-19 and spreading awareness to avoid contracting the contagion.
Notably, the U.S. federal authorities met several pharma/biotech companies this week to discuss the progress of any treatment for the Covid-19. Per a Reuters article, the Trump administration urged companies to collaborate on the accelerated development of a vaccine. Representatives from various companies at the meeting suggested 12-to-18 month timeline for a safe and tested vaccine to be available in the market.
However, antiviral treatments, which are already being developed for other viral infections, are expected to get a faster approval and may lead to earlier availability of the Covid-19 treatment. Currently, AbbVie’s (NYSE:ABBV) HIV drug, Kaletra, is being administered to coronavirus-affected patients in China. Gilead (NASDAQ:GILD) initiated two phase III studies last month to evaluate its antiviral candidate, remdesivir, as a treatment for the Covid-19. Pipeline candidates of other companies are also in early stages of development.
Earlier this week, Pfizer (NYSE:PFE) announced that it identified certain antiviral compounds, already in development, with potential to treat coronavirus.
Currently, it seems difficult for companies to bring the coronavirus disaster under control. However, we remain optimistic as several companies along with global authorities are working closely to introduce a treatment as early as possible to check this deadly virus.
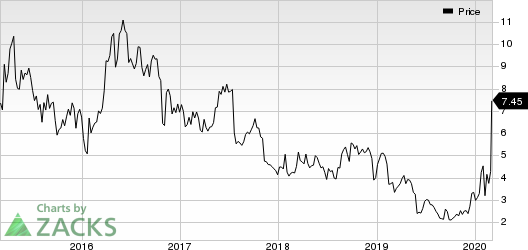
Inovio Pharmaceuticals, Inc. Price
Zacks Rank
Inovio currently carries a Zacks Rank #3 (Hold).You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2019, while the S&P 500 gained and impressive +53.6%, five of our strategies returned +65.8%, +97.1%, +118.0%, +175.7% and even +186.7%.
This outperformance has not just been a recent phenomenon. From 2000 – 2019, while the S&P averaged +6.0% per year, our top strategies averaged up to +54.7% per year.
See their latest picks free >>
Pfizer Inc. (PFE): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

• Trump’s trade war, inflation data, and last batch of earnings will be in focus this week. • DoorDash’s imminent inclusion in the S&P 500 is likely to trigger a wave of...

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.