
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Inovio (INO) Posts Wider Q4 Loss, Gets COVID-19 Vaccine Grant

Inovio Pharmaceuticals, Inc.’s (NASDAQ:INO) adjusted loss of 32 cents per share in fourth-quarter 2019 was wider than the Zacks Consensus Estimate of a loss of 23 cents.
Adjusted loss in the quarter excludes the impact of change in fair value of derivative liability and loss on investments in affiliated entities. However, including this impact, Inovio’s loss of 38 cents in the fourth quarter widened from the prior-year loss of 34 cents.
Revenues of $0.3 million in the reported quarter were down from the year-earlier quarter’s $2.5 million. Sales also missed the Zacks Consensus Estimate of $1 million.
Shares of Inovio have skyrocketed 162.4% in the past year against the industry’s decline of 15.8%.
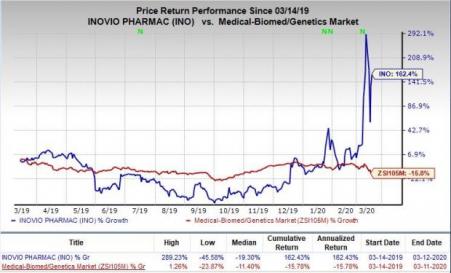
Quarter in Detail
Research and development expenses were $22 million in the fourth quarter, down 16.7% year over year, mainly due to lower employee compensation cost and drug manufacturing expenses related to Inovio’s partnership with AstraZeneca (NYSE:AZN) .
General and administrative expenses soared 55.4% to $8.7 million in the reported quarter.
Inovio had $89.5 million worth of cash, cash equivalents and short-term investments as of Dec 31, 2019 compared with $93.8 million on Sep 30, 2019.
Full-Year Results
For 2019, Inovio’s revenues of $4.1 million were down 86.5% year over year.
Loss per share was $1.21 in 2019 compared with the loss of $1.05 in 2018.
Updates on COVID-19 Vaccine Candidate
We remind investors that in January 2020, Inovio received a grant of up to $9 million from CEPI for the pre-clinical and phase I development of INO-4800, its DNA vaccine, to treat COVID-19 caused by the novel coronavirus. Currently, INO-4800 is in preclinical testing, designed to precisely match the DNA sequence of the deadly virus.
Inovio expects to begin human clinical studies on INO-4800 in April this year. Clinical studies in China and South Korea are expected to begin shortly thereafter.
Importantly, by 2020-end, Inovio plans to deliver one million doses of INO-4800 for further clinical studies and emergency use to treat the coronavirus-affected people.
In a separate press release, Inovio announced that it has received a new $5 million grant from the Bill & Melinda Gates Foundation to accelerate the testing and scale up of its proprietary CELLECTRA 3PSP smart device for the intradermal delivery of INO-4800.
Other Pipeline Updates
VGX-3100, an HPV immunotherapy, is the most advanced candidate in Inovio’s pipeline right now.
VGX-3100 is currently being evaluated in a phase III study (REVEAL 1) for the treatment of cervical dysplasia caused by papillomavirus (HPV). Top-line efficacy data from the REVEAL 1 program is expected by the fourth quarter of 2020. Another phase III study (REVEAL 2) is also on track as Inovio is recruiting patients in the United States as well as in other countries.
Inovio is also evaluating VGX-3100 in two phase II studies for the treatment of anal dysplasia caused by human papillomavirus (HPV) and for vulvar dysplasia caused by HPV. Inovio plans to report preliminary efficacy and safety data from both studies at an upcoming medical conference later in the ongoing month.
In February 2020, the FDA accepted Inovio’s investigational new drug (IND) application for its novel DNA medicine INO-3107. The regulatory body’s acceptance allows the company to begin a phase I/II study to evaluate INO-3107 for the treatment of patients with recurrent respiratory papillomatosis (RRP), a rare disease caused by certain types of HPV.
Meanwhile, in November 2019, Inovio announced positive interim data from the phase II study on its immuno-oncology combo of INO-5401 and INO-9012 in combination with Regeneron (NASDAQ:REGN) and Sanofi’s (NASDAQ:SNY) PD-1 inhibitor Libtayo (cemiplimab) for addressing newly-diagnosed patients with glioblastoma (GBM). The company plans to present 12-month overall survival data from the same in the next quarter.
This apart, the company is working on developing vaccines for Ebola, Zika, Lassa Fever and the Middle East respiratory syndrome (MERS) virus.
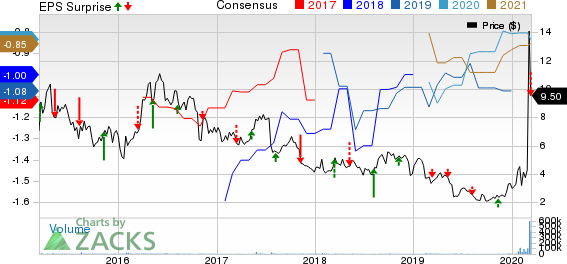
Inovio Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
Zacks Rank
Inovio currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks Top 10 Stocks for 2020
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-hold tickers for the entirety of 2020?
Last year's 2019 Zacks Top 10 Stocks portfolio returned gains as high as +102.7%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys.
Access Zacks Top 10 Stocks for 2020 today >>
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Sanofi (PA:SASY) (SNY): Free Stock Analysis Report
AstraZeneca PLC (AZN): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Original post
Related Articles

Early in 2025, value stocks emerged as a popular choice among investors seeking market-beating returns. However, factor-based investing strategies can be notoriously difficult to...

Mid-cap stocks don’t get the same headlines as large caps but move aggressively in both directions, creating outsized opportunities for investors. Unlike their mega-cap...

There’s no doubt it’s been a rough couple weeks for stocks: Both the S&P 500 and the tech-focused NASDAQ have wiped out most of this year’s gains, as of this writing. Stocks...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.