
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Idera Completes Recruitment In Late-Stage Melanoma Study

Idera Pharmaceuticals, Inc. (NASDAQ:IDRA) announced that it has completed patient enrollment in the phase III ILLUMINATE-301 study, evaluating tilsotolimod in combination with Bristol-Myers’ (NYSE:BMY) Yervoy (ipilimumab) for treating patients with anti-PD-1 refractory advanced melanoma.
Top-line overall response rate (ORR) and other preliminary data from this late-stage registrational ILLUMINATE-301 study are expected in the first quarter of 2021.
Per the company, tilsotolimod is an investigational an agonist of Toll-like receptor 9, which is being developed via intratumoral injection for addressing the given indication.
The randomized, ILLUMINATE-301 study is evaluating the effectiveness of intratumoral tilsotolimod in combination with Yervoy in patients with anti-PD-1 refractory advanced melanoma compared to Yervoy alone.
The primary endpoint of the study is ORR and the overall survival (OS) while the secondary endpoints include durable response rate, time to response, progression-free survival, safety and patient-reported outcomes.
Shares of Idera have plunged 41.9% in the past year compared with the industry’s decrease of 1.2%.
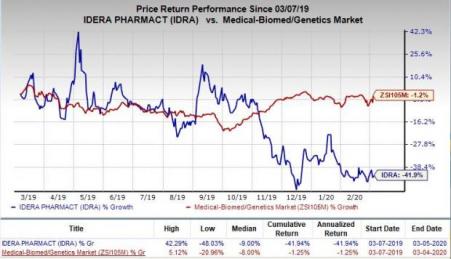
Tilsotolimod is also being evaluated in combination with several checkpoint inhibitors for treating multiple tumor types.
In January 2020, Idera announced an update on the clinical development program for tilsotolimod.
The phase II ILLUMINATE 206 study will evaluate the safety and effectiveness of tilsotolimod in combination with Yervoy and Bristol-Myers’ Opdivo (nivolumab) for the treatment of solid tumors.
Another phase I/II ILLUMINATE 204 study is designed to evaluate tilsotolimod in combination with Yervoy or Merck’s (NYSE:MRK) Keytruda (pembrolizumab) in patients with anti-PD-1 refractory metastatic melanoma. Final results from the same are expected to be announced in the second quarter of 2020.
We remind investors that in 2017, the FDA granted Fast Track designation to the combo of tilsotolimod plus Yervoy for the treatment of anti-PD-1 refractory melanoma.
Zacks Rank & Key Pick
Idera currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the biotech sector is Nabriva Therapeutics AG (NASDAQ:NBRV) , which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Nabriva’s loss per share estimates have been narrowed 3.3% for 2020 over the past 60 days.
Biggest Tech Breakthrough in a Generation
Be among the early investors in the new type of device that experts say could impact society as much as the discovery of electricity. Current technology will soon be outdated and replaced by these new devices. In the process, it’s expected to create 22 million jobs and generate $12.3 trillion in activity.
A select few stocks could skyrocket the most as rollout accelerates for this new tech. Early investors could see gains similar to buying Microsoft (NASDAQ:MSFT) in the 1990s. Zacks’ just-released special report reveals 8 stocks to watch. The report is only available for a limited time.
See 8 breakthrough stocks now>>
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Idera Pharmaceuticals, Inc. (IDRA): Free Stock Analysis Report
Nabriva Therapeutics AG (NBRV): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Using the Elliott Wave Principle (EWP), we have been tracking the most likely path forward for the Nasdaq 100 (NDX). Although there are many ways to navigate the markets and to...

Investors are on edge about what tariff policy means for markets Coming off a strong Q4 earnings season, fresh February corporate sales figures can help assess the macro...

Broadcom stock is in a dynamic rebound phase. Markets seem optimistic ahead of the earnings release. Let's take a deep dive into what to expect from the report. Get the...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.