
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Gilead's HCV Drug Epclusa Gets FDA Nod For Expanded Age Group

Gilead Sciences, Inc. (NASDAQ:GILD) announced that the FDA has approved the supplemental new drug application (sNDA) to update its hepatitis C infection (HCV) drug, Epclusa’s label. The sNDA sought approval of Epclusa for the treatment of chronic HCV in pediatric patients aged six years and above or weighing at least 17 kg, regardless of HCV genotype or liver disease severity.
Notably, Epclusa was approved in 2016 by the FDA and European Medicines Agency (EMA) for treating adult patients with chronic HCV infection. The drug is currently under review in Europe for use in pediatric patients aged six to less than 18 years.
Per the company, Epclusa is the first pan-genotypic, protease inhibitor-free regimen to be approved in the United States for adults and children to address the given indication. The recommended dosage of Epclusa is based on weight and liver function in children aged six years and above.
The approval was based on data from the open-label phase II Study 1143 study. The safety profile of Epclusa in the pediatric patient population was generally consistent with what was observed in adults.
However, the product label for Epclusa comes with a boxed warning in the United States.
Shares of Gilead have rallied 21% in the past year against the industry’s decline of 17.7%.
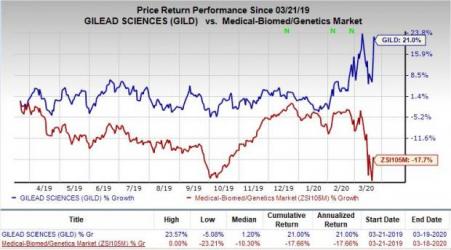
We remind investors that Gilead’s HCV franchise has witnessed a significant slowdown across key markets, including the United States and Europe. Nevertheless, Gilead remains a dominant player in the HIV market.
Meanwhile, Gilead has been in the spotlight recently as its experimental candidate remdesivir showed promise in treating patients with COVID-19. Last month, Gilead initiated two phase III studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19. These randomized, open-label, multicenter studies will enroll approximately 1,000 patients at medical centers, primarily across the Asian countries.
We note that efforts to develop a vaccine for combating the deadly novel coronavirus accelerated in the last couple of weeks. Earlier in the week, Moderna, Inc., (NASDAQ:MRNA) dosed the first participant in the phase I study of mRNA vaccine (mRNA-1273) against SARS-CoV-2. Quite a few others like Novavax, Inc. (NASDAQ:NVAX) and Inovio Pharmaceuticals, Inc. (NASDAQ:INO) are also developing vaccines to treat the disease.
Zacks Rank
Gilead currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2019, while the S&P 500 gained and impressive +53.6%, five of our strategies returned +65.8%, +97.1%, +118.0%, +175.7% and even +186.7%.
This outperformance has not just been a recent phenomenon. From 2000 – 2019, while the S&P averaged +6.0% per year, our top strategies averaged up to +54.7% per year.
See their latest picks free >>
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Moderna, Inc. (MRNA): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Since the Robotaxi event on October 11th, Tesla (NASDAQ:TSLA) stock is up 38%, currently priced at $291.60 per share This is a return to the early November 2024 price level. But...

The Q4 2024 earnings season tapers off from here, with S&P 500® EPS growth surpassing 17%, the highest in 3 years Large cap outlier earnings dates this week include:...

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.