
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Gilead (GILD) Announces Positive Long-Term Results On Descovy

Gilead Sciences, Inc. (NASDAQ:GILD) announced positive long-term results from the DISCOVER study of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets, F/TAF) for pre-exposure prophylaxis (PrEP).
These data were presented at the 2020 Conference on Retroviruses and Opportunistic Infections (CROI) in Boston.
These longer-term efficacy and safety outcomes from the DISCOVER study showed that Descovy is effective for HIV prevention with non-inferior efficacy to Truvada, and that Descovy has an improved bone and renal safety profile compared with Truvada at week 96.
We note that Descovy for PrEP is indicated to reduce the risk of sexually acquired HIV-1 infection in at-risk adults and adolescents weighing at least 35 kg, excluding individuals at risk of HIV-1 from receptive vaginal sex because effectiveness in this population has not been evaluated yet.
The above-mentioned results were achieved in the overall study population of men and transgender women at risk of HIV infection as well as study sub-population of participants aged 50 and older, those younger than 25 years and those with moderate renal impairment.
Meanwhile, a separate analysis of the DISCOVER study demonstrated that Descovy and Truvada were effective and well tolerated in Black and Hispanic/Latinx participants.
In addition, Gilead announced results from a phase Ib study evaluating the company’s investigational toll-like receptor 7 (TLR7) agonist, vesatolimod, as part of an HIV cure research program. These findings mark the first clinical data showing TLR7 stimulation by vesatolimod is associated with a modestly increased time to viral rebound compared to placebo, as well as enhanced immune function and decreased levels of intact HIV DNA.
The results presented at CROI 2020 support further evaluation of vesatolimod as part of investigational curative regimens aimed at achieving antiretroviral therapy (ART)-free control of HIV.
We note that the massive decline in sales of the HCV franchise has propelled Gilead to focus on the HIV franchise, CAR T therapy and other newer avenues. The rapid adoption of Biktarvy augments the HIV business amid stiff competition from the likes of GlaxoSmithKline (NYSE:GSK) . Gilead recently announced that it will acquire a clinical-stage immuno-oncology company, Forty Seven, Inc. (NASDAQ:FTSV) , for $95.50 per share in cash or approximately $4.9 billion.
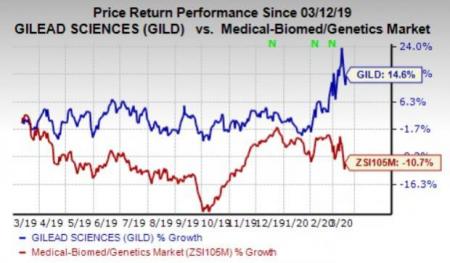
Gilead’s stock has gained 14.6% in the past year against the industry's decline of 10.7%.
The stock is in the news, lately, as Gilead announced the initiation of two-phase III studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19 (novel coronavirus).
These randomized, open-label, multicenter studies will enroll approximately 1,000 patients at medical centers primarily across Asian countries, as well as other countries globally with high numbers of diagnosed cases. The studies will assess two dosing durations of remdesivir, administered intravenously. The initiation of these studies follows the FDA’s rapid review and acceptance of Gilead’s investigational new drug (IND) filing for remdesivir for the treatment of COVID-19.
We note that quite a few companies are working on drugs and vaccines for the treatment coronavirus, as more and more cases are being reported every day. AbbVie’s (NYSE:ABBV) Kaletra is also being evaluated as a treatment option.
Gilead currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Hottest Tech Mega-Trend of All
Last year, it generated $24 billion in global revenues. By 2020, it's predicted to blast through the roof to $77.6 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Forty Seven, Inc. (FTSV): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

When looking for dividend stocks, high dividend yields are one important factor to consider. Even if a company’s dividend yield isn’t nearing double-digit percentages, finding...

Whenever Wall Street authoritative figures, such as a large institution or individual investor, decide to shift a view on a specific stock or industry, retail traders can...

Monster Beverage (NASDAQ:MNST) faces headwinds that make it a potentially scary buy, including weakness in the alcohol segment. With the alcohol business contracting in Q4 2024,...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.