
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Gilead Asks FDA To Withdraw Orphan Drug Tag For Remdesivir

Shares of Gilead Sciences, Inc. (NASDAQ:GILD) were down 5.8% after it reportedly asked the FDA to rescind the Orphan Drug designation granted to its experimental drug, remdesivir, for the treatment of COVID-19.
On Mar 23, 2020, the agency had granted remdesivir the Orphan Drug designation for the treatment of COVID-19.
Gilead had initiated two phase III studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19. These randomized, open-label, multicenter studies will enroll approximately 1,000 patients at medical centers primarily across Asian countries.
The studies will assess two dosing durations of the candidate, administered intravenously. Reportedly, remdesivir is already being used in the United States for the treatment of the disease under federal rules that allow the use of unapproved drugs on compassionate grounds.
The candidate was previously under testing for the Ebola virus. As the candidate has shown promising results in the infected patients, investors are banking on Gilead for being the first company to come up with a treatment for this deadly disease.
We note that the Orphan Drug designation is generally granted to drugs being developed for rare diseases, which affect less than 200,000 persons in the United States. The designation gives special tax and market exclusivity incentives.
However, Gilead has asked the agency to revoke the same after facing criticism to seek benefits amid a global health crisis.
The biotech was earlier accused of exorbitant pricing of its HCV drugs.
We note that there are currently no FDA-approved treatments for the severe illness caused by SARS-CoV-2. Given the alarming levels of spread and severity, some approved drugs or pipeline candidates are being tested to see if they are effective in treating infected patients.
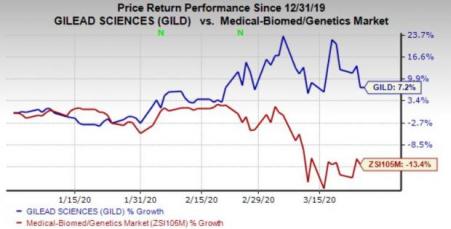
Gilead’s shares have gained 7.2% this year so far against the industry’s decline of 13.4%.
Regeneron Pharmaceuticals (NASDAQ:REGN) too has identified antibodies, which can possibly treat COVID-19. The company has now isolated hundreds of virus-neutralizing, fully-human antibodies from its VelocImmune mice, which have been genetically modified to have a human immune system.
The company has also isolated antibodies from humans who have recovered from COVID-19 to maximize the pool of potent antibodies. The company plans to select the top two antibodies for a 'cocktail' treatment based on potency and binding ability to the SARS-CoV-2 spike protein as well as other desirable qualities. Earlier,
Regeneron and partner Sanofi (NASDAQ:SNY) announced a program to evaluate their rheumatoid arthritis (RA) drug, Kevzara, to treat patients hospitalized with severe infection due to COVID-19.
President Trump reportedly wants to speed up the approvals of vaccines and treatments to fight the pandemic.
Earlier this week, Roche (OTC:RHHBY) obtained FDA nod to conduct a double-blind, placebo-controlled phase III study to evaluate the safety and efficacy of intravenous rheumatoid arthritis (RA) drug, Actemra (tocilizumab), plus standard of care in hospitalized adult patients with severe COVID-19 pneumonia.
Desperate times call for desperate measures and pharma/biotech companies are running a race against time to successfully develop treatments and vaccines to combat this contagious disease. While the drugs and vaccines will need some time to be tested and a cure is not imminent, investors will keep an eye on these companies as the pandemic is not likely to die out soon.
Gilead currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +24.5% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Sanofi (SNY): Free Stock Analysis Report
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.