
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

FDA EUA For Coronavirus Tests Boosts MedTech: 3 Stocks In Focus

The coronavirus pandemic continues to weigh on the global stock market.Most U.S. companies across industry domains have slashed their first-quarter guidance owing to expectations of revenue losses. Last month, a study by The Guardian estimated that the outbreak might cost the global economy more than $1 trillion.
FDA Steps Up Efforts
Amid this crisis, the FDA has been continuously stepping up its efforts to bring swift and efficient solutions, which can bring respite to patients and healthcare professionals. On Feb 29, the FDA decided to allow certain certified laboratories to urgently begin developing validated coronavirus diagnostics, subject to the condition that the test is submitted for review within 15 days to the federal agency.
Several key biotech and molecular diagnostic players received FDA’s Emergency Use Authorization (EUA) for COVID-19 tests, which they have been rigorously working on developing and launching commercially lately. Through this, the federal regulatory body expedited the approval procedure for the companies’ tests so that diagnostic professionals could begin conducting the same in any state health laboratory immediately.
Recently Mesa Biotech attained FDA’s EUA for its point-of-care COVID-19 test. This comes right on the heels of diagnostics firm Cepheid attaining the same for its rapid molecular diagnostic test, Xpert Xpress SARS-CoV-2.
Quest Diagnostics (NYSE:DGX) too is set to launch a COVID-19 test service. This test would facilitate the potential detection of nucleic acid in respiratory specimens of patients who fulfill CDC's clinical criteria for COVID-19 testing. However, the FDA’s review under EUA is still pending. Hologic (NASDAQ:HOLX) too announced the receipt of the FDA’s EUA for its latest molecular diagnostic test, Panther Fusion SARS-CoV-2 assay, a real-time polymerase chain reaction (RT-PCR) in-vitro diagnostic test, which is intended to be used for the qualitative detection of RNA from the SARS-CoV-2.
3 Stocks to Focus On
The following Zacks Rank #3 (Hold) companies have already attained EUA for their newly developed coronavirus tests, banking on which the stocks are currently outperforming the broader industries they belong to.You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here
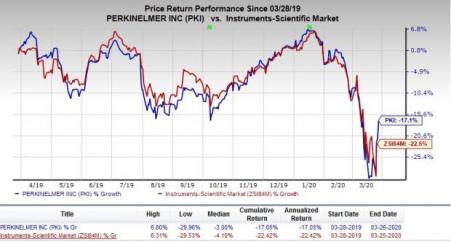
PerkinElmer ( (NYSE:PKI) ): This Zacks Rank #3 company recently attained EUA from the FDA for its New Coronavirus RT-PCR test. Clinical laboratories that are certified under Clinical Laboratory Improvement Amendments (CLIA) can start utilizing this test kit to detect SARS-CoV-2 (virus causing COVID-19) immediately. Notably, this test is marketed as an in-vitro diagnostic (IVD) device on basis of fulfilling the requirements of European In Vitro Diagnostic Directive (IVDD). The test kit is now available in more than 30 countries worldwide.
In the past year, the company’s shares have outperformed the industry. The stock has lost 17.1% compared with the industry’s 22.5% decline.
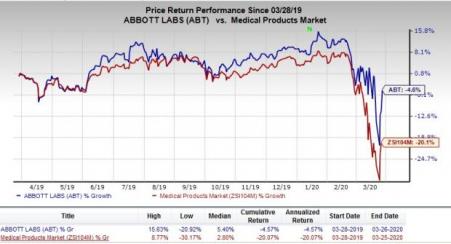
Abbott Laboratories ( (NYSE:ABT) ) : This Zacks Rank #3 global major recently announced the receipt of the EUA from the FDA to use its molecular test RealTime SARS-CoV-2 EUA to detect (COVID-19. Notably, the test will run on the company’s m2000 RealTime System. Abbott is on track to ship 150,000 RealTime SARS-CoV-2 EUA tests to existing customers in the United States. At the same time, the company will coordinate with health systems and government authorities to supply additional m2000 systems per requirement.
In the past year, the company’s shares have outperformed the industry. The stock has lost 4.6% compared with the industry’s 20.1% decline.
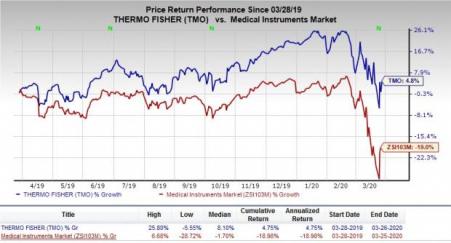
Thermo Fisher (NYSE:TMO) : This Zacks Rank #3 global manufacturer of scientific instruments recently attained EUA from the FDA for its diagnostic test to be used immediately by CLIA high-complexity laboratories in the United States. The test has been developed for the detection of nucleic acid exclusively from SARS-CoV-2. The authorized test utilizes the Applied Biosystems TaqPath Assay technology and has been developed to deliver patient outcomes within four hours of a sample reaching a laboratory. The estimated time-to-result constitutes of time for sample preparation and instrument analysis. The EUA test is optimized for use on the company's Applied Biosystems 7500 Fast Dx Real-time PCR instrument. The instrument is covered under the EUA and is already utilized in clinical laboratories across the globe.
In the past year, the company’s shares have outperformed the industry.The stock has rallied 4.8% against the industry’s 19% decline.
The Hottest Tech Mega-Trend of All
Last year, it generated $24 billion in global revenues. By 2020, it's predicted to blast through the roof to $77.6 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Quest Diagnostics Incorporated (DGX): Free Stock Analysis Report
Hologic, Inc. (HOLX): Free Stock Analysis Report
PerkinElmer, Inc. (PKI): Free Stock Analysis Report
Abbott Laboratories (ABT): Free Stock Analysis Report
Thermo Fisher Scientific Inc. (TMO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.