
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Editas, Allergan Start Dosing In Early-Stage Eye Disease Study

Editas Medicine, Inc. (NASDAQ:EDIT) and Allergan plc (NYSE:AGN) announced that they have dosed the first patient in a phase I/II study — BRILLIANCE — evaluating their CRISPR-based candidate, AGN-151587 (EDIT-101), in patients with Leber congenital amaurosis 10 (LCA10), an inherited form of blindness.
Enrollment in the BRILLIANCE study was initiated in July 2019. The study is the first to evaluate a CRISPR genome editing medicine in vivo, or inside the body in LCA10 patients. The five-cohort study will evaluate three dose levels of AGN-151587 in pediatric as well as adult patients. AGN-151587 is the lead candidate in Editas’ pipeline.
The CRISPR-based candidate was developed by Editas as part of a strategic alliance and option agreement with Allergan. The agreement grants Allergan option to license up to five of Editas Medicine’s genome editing programs for ocular diseases including AGN-151587. Allergan exercised its option to gain exclusive rights to AGN-151587 in 2018. Please note that Allergan and Editas are co-developing the candidate and will share profit/loss equally from any sales in the United States, following its successful development.
Share of Editas have declined 3.4% in the past year compared with the industry’s decrease of 9.7%.
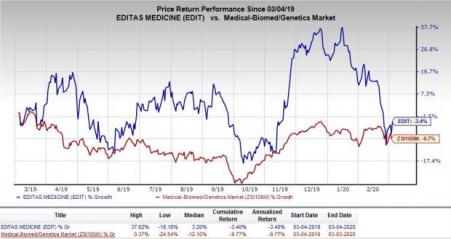
Currently, there are no approved therapies for treating LCA10. With a significant unmet need, AGN-151587 can reap huge profits, if developed successfully and eventually approved. However, another company, ProQR Therapeutics N.V. (NASDAQ:PRQR) is also conducting a clinical study for treating LCA10 with its own product candidate. The company initiated a phase II/III study on its candidate last year. Data from the study can be used for filing regulatory application seeking the candidate’s approval. An earlier approval to ProQr’s candidate will likely have an unfavorable impact on AGN-151587’s prospects.
Editas is also pursuing the development of CRISPR candidates for eye diseases other than LCA10 including Usher Syndrome type 2A (USH2A) and the recurrent ocular Herpes Simplex Virus type 1 (HSV-1) fibrosis. It is also designing novel medicines for non-malignant hematologic diseases, such as sickle cell disease and beta-thalassemia.
Meanwhile, Editas has some high-profile collaborations with big pharma entities for its CRISPR technology, which provide research support and sufficient funds to fulfill its pipeline development plans. Apart from Allergan, it has a collaboration and licensing pact with another big pharma company, Celgene (NASDAQ:CELG) — now part of Bristol-Myers (NYSE:BMY) — to use the latter’s gene-editing approaches including CRISPR-Cas9 for developing the engineered T cell medicines to tackle cancer.
Such collaboration deals provide regular funds to Editas for executing its pipeline activities.
Zacks Rank
Editas currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Free: Zacks’ Single Best Stock Set to Double
Today you are invited to download our latest Special Report that reveals 5 stocks with the most potential to gain +100% or more in 2020. From those 5, Zacks Director of Research, Sheraz Mian hand-picks one to have the most explosive upside of all.
This pioneering tech ticker had soared to all-time highs and then subsided to a price that is irresistible. Now a pending acquisition could super-charge the company’s drive past competitors in the development of true Artificial Intelligence. The earlier you get in to this stock, the greater your potential gain.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Allergan plc (AGN): Free Stock Analysis Report
Editas Medicine, Inc. (EDIT): Free Stock Analysis Report
ProQR Therapeutics N.V. (PRQR): Free Stock Analysis Report
Original post
Related Articles

• Trump’s trade war, inflation data, and last batch of earnings will be in focus this week. • DoorDash’s imminent inclusion in the S&P 500 is likely to trigger a wave of...

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.