
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

CytomX & Astellas Collaborate To Develop Novel Cancer Therapy

CytomX Therapeutics, Inc. (NASDAQ:CTMX) and Japan-based Astellas Pharma entered into a strategic collaboration for discovering, developing and commercialization of novel T-cell engaging bispecific antibodies, which target CD3 and tumor cell surface antigens for treating cancer. The companies will use CytomX’s Probody therapeutic technology platform and bispecific formats and CD3 modules for development of treatment targeting solid tumors.
Per the terms of the deal, Astellas will pay CytomX an upfront payment of $80 million in cash. CytomX will also be eligible to receive additional payments of up to $1.6 billion related to future preclinical, clinical and commercial milestones as well as royalties on global net sales on any commercialized product under the deal. CytomX will be responsible for initial research and discovery activities. Following candidate selection for development, Astellas will be responsible for preclinical and clinical development and commercialization activities.
Meanwhile, CytomX has options to co-develop a specified number of targets with Astellas Pharma, which need to be exercised before initiation of first pivotal study on chosen candidates. CytomX will be eligible to receive certain portion of profits from sales of jointly developed drugs in the United States. The company may also elect to co-commercialize the products in the U.S. market.
The deal gives CytomX the opportunity to expand its potentially transformational Probody technology and provides the company with additional financial and strategic flexibility.
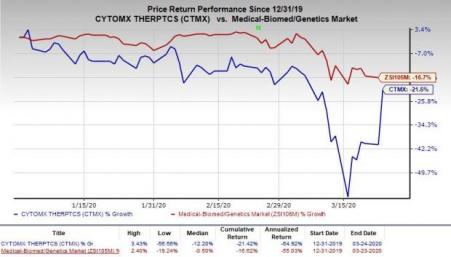
So far this year, share of CytomX have declined 21.5% compared with the industry’s decrease of 16.7%.
Apart from Astellas Pharma, CytomX has other collaboration agreements with multiple pharma/biotech companies related to use of its Probody therapeutic technology platform. The company is developing two early- to mid-stage candidates in partnership with Bristol-Myers (NYSE:BMY) in combination with the latter’s PD-1 inhibitor, Opdivo, targeting cancer indication. CytomX is also developing another early-stage candidate in collaboration with AbbVie (NYSE:ABBV) targeting solid tumors. Last year, AbbVie selected a second research target to develop under the collaboration. CytomX also has a collaboration with Amgen (NASDAQ:AMGN) to develop CytomX Probody T-cell engaging bispecific program that targets the EGFR protein in cancerous cell.
CytomX also has two candidates in its internal pipeline. The lead candidate, CX-072, a Probody therapeutic targeting PD-L1, is being developed as monotherapy in five oncology indications including triple negative breast cancer. The company is developing CX-072 in combination with Bristol-Myers’ Yervoy in patients with unresectable or metastatic melanoma whose disease has progressed or relapsed after receiving treatment with a PD-1/PD-L1 immune checkpoint inhibitor. The candidate is also being developed in combination with Roche’s cancer drug, Zelboraf, in patients with V600E BRAF-positive melanoma.
CytomX is developing the second candidate, CX-2009, as monotherapy in a phase II study as a treatment for hormone receptor (ER, PR) positive, HER2 negative breast cancer.
Zacks Rank
CytomX currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
CytomX Therapeutics, Inc. (CTMX): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.