
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Coronavirus Vaccine Sales Key to Moderna's (MRNA) Q3 Earnings

Moderna (NASDAQ:MRNA), Inc. MRNA received the first emergency use authorization (“EUA”) for its COVID-19 vaccine, mRNA-1273, for adults in December 2020 in the United States. The vaccine has boosted the company’s top line significantly in the past two quarters. The Zacks Consensus Estimate for third-quarter revenues is pegged at $6.09 billion.
During the third quarter, a booster or “third” dose of the company’s COVID-19 vaccine was authorized for use in immunocompromised adults in the United States and Europe. The initial two-dose regimen of the vaccine was authorized for use in adolescents in Europe during the quarter as well. The vaccine was also authorized for use in adults in a few new countries in the third quarter. These are likely to have aided sales in the soon-to-be reported quarter.
The booster dose of mRNA-1273 was authorized in the United States last month for use in adults at high risk of COVID-19 infections. The company may provide an updated guidance to include future revenues from booster doses on its third-quarter earnings call.
Moderna is seeking EUA for the use of its vaccine adolescents in the United States. However, the FDA had notified last week that additional time would be required to review the EUA request for evaluating the increased risk of myocarditis related to the vaccine. Moderna decided to delay its filing of a EUA request for the use of its vaccine in children aged 6 to less than 12 years. Investors are likely to ask questions on the impact of these delays on near-term growth.
We note that Pfizer PFE, in collaboration with BioNTech BNTX, J&J and AstraZeneca (NASDAQ:AZN) AZN are companies with an authorized/approved coronavirus vaccine. Several other vaccines for COVID-19 are in development and are expected to get approval soon. The company may discuss competition following the anticipated delay in getting EUA for the new population on its third-quarter earnings call.
The company has received a commitment of approximately $1.3 billion from the U.S. government's Operation Warp Speed program and BARDA. These funds will be available to the company as and when needed as grants or awards. The company used a significant amount of funding and was left with $421 million at the end of the second quarter. The company may have used the remaining fund to submit different EUA requests and a complete approval request for mRNA-1273 in the United States. The Zacks Consensus Estimate for grant revenues is pegged at $82 million.
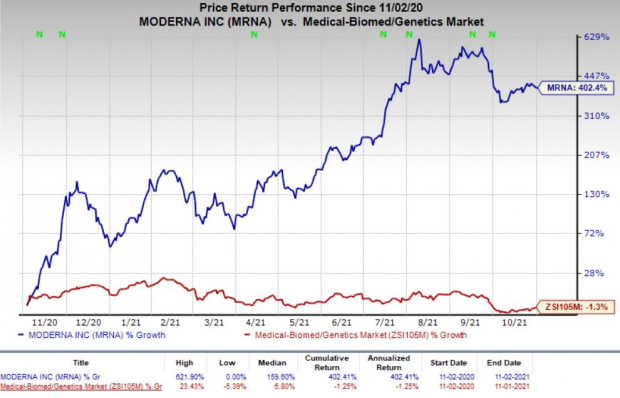
Moderna’s stock has skyrocketed 402.4% so far this year against a decrease of 1.3% for the industry.
Moderna has a few strategic alliances with pharma companies for the development of some of its pipeline candidates. These alliances generate collaboration revenues for the company. The Zacks Consensus Estimate for collaboration revenues is pegged at $16.55 million for the third quarter.
Apart from mRNA-1273, Moderna is engaged in developing several other mRNA-based products targeting a wide variety of diseases. During the third quarter, the company initiated three new phase I/II studies to evaluate a flu vaccine candidate, an autoimmune therapeutic candidate and an investigational mRNA therapeutic for methylmalonic acidemia. The company initiated a phase III study in October to evaluate its cytomegalovirus vaccine candidate. The company is also conducting clinical studies on its COVID-19 vaccine to develop it for new population or variant-specific booster doses. The company is also focused on capacity expansion to support manufacturing and supply of its COVID-19 vaccine and booster doses, and other mRNA-based pipeline candidates.
These ongoing clinical studies, preparation for the late-stage CMV vaccine study, and capacity expansion are likely to have driven operating expense higher for the company during the third quarter. (Read more: Moderna to Report Q3 Earnings: What's in the Cards?)
Zacks Rank
Moderna currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN): Free Stock Analysis Report
Pfizer Inc. (NYSE:PFE): Free Stock Analysis Report
Moderna, Inc. (MRNA): Free Stock Analysis Report
BioNTech SE Sponsored ADR (NASDAQ:BNTX): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
Related Articles

• Trump’s trade war, inflation data, and last batch of earnings will be in focus this week. • DoorDash’s imminent inclusion in the S&P 500 is likely to trigger a wave of...

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.