
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Clovis (CLVS) Q4 Loss Narrower Than Expected, Revenues Miss

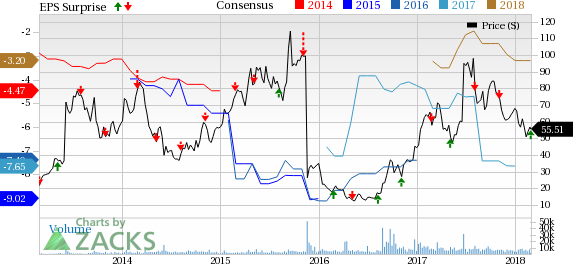
Clovis Oncology, Inc. (NASDAQ:CLVS) incurred an adjusted loss of $1.27 per share in the fourth quarter of 2017, which was narrower than the year-ago loss of $1.83 per share as well as the Zacks Consensus Estimate of a loss of $1.28.
Clovis’ only marketed drug, Rubraca, was granted accelerated approval by the FDA in December 2016 for the treatment of advanced ovarian cancer in patients who have received prior chemotherapies.
Net product revenues, entirely from Rubraca, were approximately $16.8 million in the quarter, up a mere 1.2% sequentially. However, revenues missed the Zacks Consensus Estimate of $19.22 million. Importantly, the company registered 1400 new patients on Rubraca therapy since its approval. In the fourth quarter, 300 new patients were registered. Presumably, adoption of Rubraca was slower due to its approval only in patients population who have BRCA mutation and increased competition from recently approved maintenance therapies including Tesaro, Inc.’s (NASDAQ:TSRO) Zejula and AstraZeneca PLC’s (NYSE:AZN) Lynparza.
In the year-ago quarter, Clovis had generated revenues of $0.08 million.
Shares of the company declined almost 1% in after-market trading on Monday on lower-than-expected Rubraca sales. Moreover, Clovis has significantly underperformed the industry in the past year. While the stock has lost 8.9%, the industry declined 2.5%.
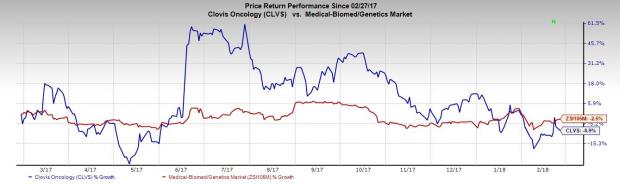
Quarter in Detail
During the fourth quarter, research & development expenses decreased 30.2% year over year to $38 million, primarily due to decreased development activities related to Rubraca and rociletinib programs. However, selling, general and administrative (SG&A) expenses escalated 216% year over year to $38.5 million, reflecting increased activities to support commercialization of Rubraca.
Cash used in operating activities in the quarter was $65.6 million, higher than $54.7 million in the year-ago quarter.
Clovis ended the quarter with $563.7 million of cash equivalents and available-for-sale securities supported by the proceeds raised through share offerings made in January and June in 2017.
2017 Performance
Product sales for the full year were $55.5 billion. Rubraca had generated sales of $0.08 in the year-ago period, after it was launched in December 2016. Adjusted loss per share narrowed 34.2% year over year to $5.12.
Update on Rubraca
The supplemental new drug application (sNDA) for Rubraca seeking label expansion as a maintenance treatment for patients with platinum-sensitive recurrent ovarian cancer was granted priority review in December 2017. The sNDA was submitted based on positive data from ARIEL 3 confirmatory study. A decision is expected in April 2018.
After ARIEL 3, another confirmatory study – ARIEL4 – is evaluating Rubraca versus chemotherapy in patients who have failed two prior lines of therapy and is open for enrolment.
Rubraca is also under review in the EU for advanced ovarian cancer indication with BRCA mutation. The Committee for Medicinal Products for Human Use is expected to provide a decision in March 2018. Investors can expect a positive recommendation as the CHMP communicated a positive trend vote last week. Additionally, Clovis has plans to submit a supplemental application seeking approval for second-line or later maintenance treatment indication in the EU, only if approved for the advanced ovarian cancer indication.
In July 2017, Clovis announced a collaboration with Bristol-Myers Squibb Company (NYSE:BMY) to evaluate Rubraca in combination with the latter’s PD-1 immune checkpoint inhibitor, Opdivo, in multiple tumor types. The companies initiated a phase II study in December 2017 to evaluate the combination in patients with metastatic castrate-resistant prostate cancer.
Clovis is also evaluating or planning to develop Rubraca in several studies to expand its label into additional indications like prostrate, breast and pancreatic cancers, among others either as monotherapy or in combination with other agents.
Zacks Rank
Clovis has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Zacks Top 10 Stocks for 2018
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-hold tickers for the entirety of 2018?
Last year's 2017 Zacks Top 10 Stocks portfolio produced double-digit winners, including FMC Corp (NYSE:FMC). and VMware which racked up stellar gains of +67.9% and +61%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys.
Access Zacks Top 10 Stocks for 2018 today >>
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
TESARO, Inc. (TSRO): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.