
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Celldex (CLDX) Initiates Phase II Study On Cancer Candidate

Celldex Therapeutics, Inc. (NASDAQ:CLDX) announced the initiation of a phase II study on its human monoclonal antibody, CDX-3379 in patients with advanced head and neck squamous cell carcinoma ("HNSCC").
With no approved products in its portfolio, investor focus remains on the progress of Celldex’s pipeline candidates.
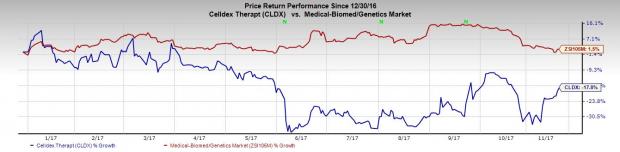
Shares of the company were up more than 2% on Friday following the news. However, Celldex’s shares were down 17.8% so far this year, underperforming the industry’s gain of 1.5% in that period.
The phase II study will evaluate CDX-3379 in combination with Eli Lilly’s (NYSE:LLY) Erbitux in patients with advanced HNSCC who are Erbitux resistant and previously treated with an anti-PD1 checkpoint inhibitorThe company believes that the patient population being evaluated in the study has limited availability of treatment options and a particularly poor prognosis. The study will continue the treatment till progression of the disease starts or patients become intolerant to the combination therapy. The primary objective of the study is to achieve objective response rate.
The two-stage study will enroll 13 patients in the first stage and initiate the second stage upon achieving partial or complete response in at least one patient.
We remind investors that a completed phase Ib study on the combination therapy had shown promising anti-tumor activity in HSNCC patients including a durable complete response in a single patient whose disease had progressed on Erbitux monotherapy.
Celldex is also progressing well with its lead pipeline candidate, glembatumumab vedotin, which is being evaluated in a phase IIb study in patients with triple negative breast cancer and in a phase II study in melanoma patients. The company is also evaluating the candidate in combination with Bristol-Myers’s (NYSE:BMY) Opdivo or Merck’s (NYSE:MRK) Keytruda in two separate cohorts in its melanoma study.
Celldex currently holds a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Celldex Therapeutics, Inc. (CLDX): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

These stocks provide a compelling case as safe-haven stocks in the face of an escalating trade war. Each company operates within sectors that are relatively resilient to economic...

When the market narrative becomes too widely accepted, excess seems to be created in some areas of the economy as businesses prepare for what’s coming their way. Today’s stock...

Markets are bouncing back as investors bet on technical support, tariff relief, and Germany’s stimulus plans. But with ISM and NFP data ahead, Fed rate cut bets could shift,...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.