
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Catalyst Initiates Phase II Study For Pipeline Drug Firdapse

Catalyst Pharmaceuticals, Inc. (NASDAQ:CPRX) announced the initiation of phase II proof-of-concept study determining the safety, tolerability and efficacy of its lead pipeline candidate, Firdapse (amifampridine phosphate) as a symptomatic treatment for Spinal Muscular Atrophy (SMA) Type 3. Top-line results from this randomized (1:1), double-blind, 2-period, 2-treatment, crossover, outpatient proof-of-concept trial is expected in the first half of 2019.
If the outcome is positive, the company plans to submit an application for orphan drug designation and pursue a clinical program required for the filing. According to some studies, animals or patients affected with SMA have shown that the neuromuscular junction (NMJ) has significant structural and functional defects suggesting that impaired NMJ function might contribute to SMA pathogenesis and symptoms. Firdapse increases neuromuscular junction transmission and may therefore help to preserve the NMJ and motor neurons. The company thus feels that treatment with Firdapse will provide symptomatic relief to SMA Type 3 patients.
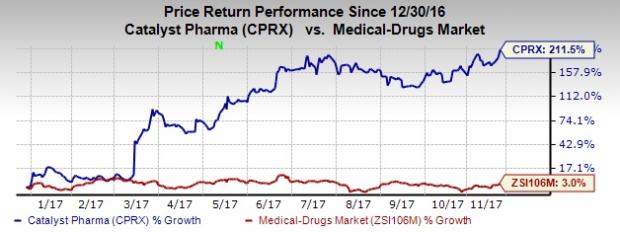
So far this year, Catalyst Pharma’s shares have outperformed the industry. The stock has surged 211.5% compared with the industry’s gain of 3%.
Firdapse is the lead pipeline candidate for the company. Catalyst had in-licensed rights to Firdapse from BioMarin Pharmaceutical Inc. (NASDAQ:BMRN) for its development and commercialization in the United States. Currently, the candidate is approved in the EU for the symptomatic treatment of Lambert-Eaton myasthenic syndrome (LEMS) in adults. Also, it enjoys Orphan Drug and Breakthrough Therapy status in the United States for the treatment of LEMS.
In December 2017, Catalyst expects to report top-line results from its second phase III study of Firdapse for the treatment of LEMS. Additionally, the company plans to resubmit a new drug application (NDA) before the end of the year.
Meanwhile, Catalyst is also working on the development of Firdapse for additional indications. The company expects to initiate MuSK- myasthenia gravis (MG) phase III study in the first quarter of 2018.
Earlier, the company had initiated a small blinded study on Firdapse for the treatment of certain types of congenital myasthenic syndromes (CMS) in pediatric population (2-17 years). However, following discussions with the FDA, the study has been expanded beyond pediatric patients to include adult CMS patients. Top-line data from the study are expected in the first half of 2018. Positive data would allow Catalyst to add the CMS indication to Firdapse’s label (either as part of the NDA resubmission for Firdapse to treat LEMS or as a supplement to the resubmission).
The company’s efforts to develop Firdapse appear encouraging, given its significant commercial potential. An approval for the same is likely to be a huge growth driver for Catalyst.
Zacks Rank & Stocks to Consider
Catalyst carries a Zacks Rank #3 (Hold). Some better-ranked health care stocks in the same space are Sucampo Pharmaceuticals (NASDAQ:SCMP) andLigand Pharmaceuticals (NASDAQ:LGND) . While Sucampo sports a Zacks Rank #1 (Strong Buy), Ligand holds a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Sucampo’searnings per share estimates have moved up from $1.01 to $1.12 for 2017 and from $1.06 to $1.19 for 2018, over the last 30 days. The company delivered a positive earnings surprise in three of the trailing four quarters, with an average beat of 15.63%.
Ligand’s earnings per share estimates have climbed $3.68 to $3.70 for 2018 over the last 60 days. The company pulled off a positive earnings surprise in two of the trailing four quarters, with an average beat of 8.22%. The share price of the company has increased 27.9% year to date.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
BioMarin Pharmaceutical Inc. (BMRN): Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND): Free Stock Analysis Report
Sucampo Pharmaceuticals, Inc. (SCMP): Free Stock Analysis Report
Catalyst Pharmaceuticals, Inc. (CPRX): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Using the Elliott Wave Principle (EWP), we have been successfully tracking the most likely path forward for the S&P 500 (SPX) over several months. Although there are many ways...

When looking for dividend stocks, high dividend yields are one important factor to consider. Even if a company’s dividend yield isn’t nearing double-digit percentages, finding...

Whenever Wall Street authoritative figures, such as a large institution or individual investor, decide to shift a view on a specific stock or industry, retail traders can...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.