
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Bristol Myers (BMY) Clinches FDA Nod For MS Drug Zeposia

Bristol-Myers Squibb Company (NYSE:BMY) announced that the FDA has approved Zeposia (ozanimod) for the treatment of adults with relapsing forms of multiple sclerosis (RMS) including those with clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease.
Per the company, the once-daily oral medicine Zeposia (0.92 mg) becomes the only-approved sphingosine-1-phosphate (S1P) receptor modulator that offers RMS patients an initiation with no genetic test and requires no label-based first-dose observation for patients.
Notably, this is the first FDA-approved new drug application for Bristol-Myers following the acquisition of biotech giant Celgene Corporation (NASDAQ:CELG) last November. The combined company is well-positioned to address the needs of patients with cancer, inflammatory, immunologic, cardiovascular or fibrotic diseases through high-value innovative medicines and leading scientific capabilities.
Shares of Bristol-Myers have rallied 9.2% in the past year against the industry’s decline of 14.5%.
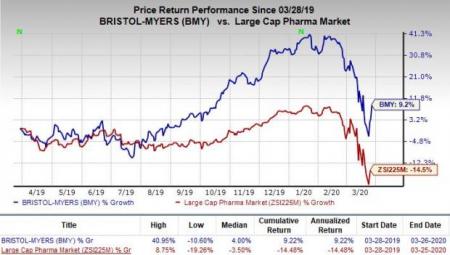
The FDA nod was based on data from the phase III SUNBEAM study and the pivotal RADIANCE Part B phase III study.
Both studies evaluated the efficacy, safety and tolerability of Zeposia against the weekly intramuscular Avonex (interferon beta-1a). While SUNBEAM investigated the same for a minimum 12-month treatment period, the other study evaluated the same for a regime of at least 24 months.
In both studies, Zeposia delivered powerful efficacy as measured by an annualized relapse rate (ARR) as well as in terms of the number and size of brain lesion compared with Avonex in the given patient population.
Zeposia is also under review in the EU for the treatment of adults with relapsing-remitting multiple sclerosis and a decision from the European Medicines Agency (EMA) is expected in the first half of 2020.
However, Bristol-Myers decided to delay the commercialization of Zeposia in the United States in the wake of the unprecedented COVID-19 pandemic, which ravaged the world of late.
The coronavirus pandemic, which claimed more than 23,000 lives globally, compelled healthcare systems to prioritize caring for the COVID-19 patients while restricting other activities. Along the same lines, earlier this week, Eli Lilly (NYSE:LLY) announced that it is halting enrollment in most ongoing studies and also decides to delay any new study starts in order to allow doctors and healthcare facilities to focus solely on efforts for combating the coronavirus disease (COVID-19). Many others are expected to follow suit.
Zacks Rank & Other Stocks to Consider
Bristol-Myers currently carries a Zacks Rank #2 (Buy). Other stocks worth considering in the large cap pharma sector include Pfizer Inc. (NYSE:PFE) and AbbVie Inc. (NYSE:ABBV) , both sporting the same Zacks Rank as Bristol-Myers. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Pfizer’s earnings estimates have been revised 4.9% upward for 2020 over the past 60 days.
AbbVie’s earnings estimates have moved 2% north for 2020 over the past 60 days.
The Hottest Tech Mega-Trend of All
Last year, it generated $24 billion in global revenues. By 2020, it's predicted to blast through the roof to $77.6 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Pfizer Inc. (PFE): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.