
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Biotech Stock Roundup: GILD To Buy Forty Seven, Pipeline Updates From BIIB & More

As the earnings season comes to an end, the focus is back on pipeline updates from biotech players. Quite a few companies came out with important news this week. Biotech bigwig, Gilead Sciences, Inc., (NASDAQ:GILD) is all set to acquire immuno-oncology company, Forty Seven. Biogen (NASDAQ:BIIB) has entered into a collaboration deal with Sangamo (NASDAQ:SGMO) . Alnylam (NASDAQ:ALNY) gets approval for Givlaari in Europe.
Recap of the Week’s Most Important Stories:
Gilead to Buy Forty Seven: Gilead Sciences, Inc. will acquire a clinical-stage immuno-oncology company, Forty Seven, Inc. (NASDAQ:FTSV) , for $95.50 per share in cash or approximately $4.9 billion. The acquisition will add Forty Seven’s investigational lead product candidate, magrolimab, to Gilead’s immuno-oncology research and development portfolio. Magrolimab is a monoclonal antibody in clinical development for the treatment of several cancers, including myelodysplastic syndrome (MDS), acute myeloid leukemia (AML) and diffuse large B-cell lymphoma (DLBCL), for which new, transformative medicines are urgently needed. The acquisition, expected to close in the second quarter of 2020, should bolster Gilead’s efforts to develop its oncology portfolio.
Alnylam’s Givlaari Gets Approval in Europe: Alnylam Pharmaceuticals Inc. announced that the European Commission has approved Givlaari (givosiran) for the treatment of acute hepatic porphyria (AHP) in patients aged 12 years or older. The drug will be available as a subcutaneous injection. The approval in Europe was in the cards as the drug had received a positive recommendation from the Committee for Medicinal Products for Human Use of the European Medicines Agency in January 2020. The approval was based on impressive data from the phase III ENVISION study, which showed that treatment with Givlaari resulted in a 74% reduction in the rate of porphyria attacks. The FDA already approved Givlaari in November last year for the same indication.
Biogen Collaborates With Sangamo: Biogen entered into a global licensing collaboration agreement with Sangamo Therapeutics, Inc. The companies are collaborating to develop and commercialize ST-501 for tauopathies, including Alzheimer’s disease; ST-502 for synucleinopathies, including Parkinson’s disease; and a third undisclosed neuromuscular disease target. Additionally, Biogen has exclusive rights to nominate up to nine additional undisclosed neurological disease targets over a target selection period of five years. The companies will leverage Sangamo’s proprietary zinc finger protein (ZFP) technology delivered via adeno-associated virus (AAV) to modulate the expression of key genes involved in neurological diseases. Biogen will pay Sangamo an upfront fee of $350 million, including a license fee and an equity investment in the Sangamo stock. Sangamo is also eligible to receive up to $2.37 billion in potential milestones as well as royalties on potential net commercial sales.
Biogen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Biohaven Gets FDA Approval for Migraine Drug: Biohaven Pharmaceutical Holding Company Ltd. (NYSE:BHVN) announced that the FDA has approved Nurtec ODT (rimegepant) for the acute treatment of migraine in adults. The FDA approval was based on results from the pivotal phase III clinical study (Study 303) and the long-term, open-label safety study (Study 201). Results showed that Nurtec ODT achieved statistical significance on the regulatory co-primary endpoints of pain freedom and freedom from most bothersome symptom (MBS) at two hours post the dose compared to placebo. Per the company, Nurtec is the first and only calcitonin gene-related peptide (CGRP) receptor antagonist available in a fast-acting orally disintegrating tablet (ODT).
Incyte & MorphoSys Get Antitrust Clearance: Incyte Corporation (NASDAQ:INCY) and MorphoSys AG announced that their collaboration and license agreement for the further development and global commercialization of the latter’s investigational candidate, tafasitamab (MOR208), has received antitrust clearance. This triggers a $750-million upfront payment to be received by MorphoSys from Incyte. Additionally, Incyte will make an equity investment of $150 million in new American Depositary Shares (ADS) in MorphoSys within the defined timelines. The FDA recently accepted MorphoSys' Biologics License Application (BLA) for tafasitamab in combination with lenalidomide for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL) and granted Priority Review.
Performance
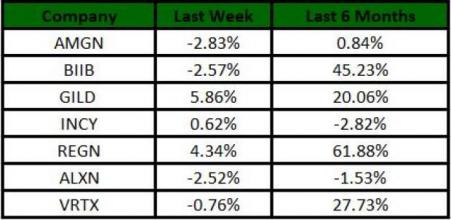
The Nasdaq Biotechnology index lost 0.51% in the last five trading sessions. Among the biotech giants, Gilead gained 5.86% during this period. Over the past six months, shares of Biogen have gained 45.23%. (See the last biotech stock roundup here: Biotech Stock Roundup: MRNA Surges, Pipeline Updates From INCY, HRTX & NGM Bio).
What's Next in Biotech?
Stay tuned for more pipeline updates.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2019, while the S&P 500 gained and impressive +53.6%, five of our strategies returned +65.8%, +97.1%, +118.0%, +175.7% and even +186.7%.
This outperformance has not just been a recent phenomenon. From 2000 – 2019, while the S&P averaged +6.0% per year, our top strategies averaged up to +54.7% per year.
See their latest picks free >>
Biogen Inc. (BIIB): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Sangamo Therapeutics, Inc. (SGMO): Free Stock Analysis Report
Biohaven Pharmaceutical Holding Company Ltd. (BHVN): Free Stock Analysis Report
Forty Seven, Inc. (FTSV): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

• Trump’s trade war, inflation data, and last batch of earnings will be in focus this week. • DoorDash’s imminent inclusion in the S&P 500 is likely to trigger a wave of...

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.