
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

BioMarin's (BMRN) Gene Therapy Enters First Phase III Study

BioMarin Pharmaceutical Inc. (NASDAQ:BMRN) announced initiation of the first phase III study, evaluating the 6e13 vg/kg dose of its investigational gene therapy, valoctocogene roxaparvovec, for treatment of patients with severe hemophilia A. The company plans to enroll the first patient in the second phase III study with the 4e13 vg/kg dose at the beginning of 2018 for the given indication.
The two phase III studies, GENEr8-1 and GENEr8-2, were designed to evaluate the efficacy and safety of valoctocogene roxaparvovec in patients with severe hemophilia A across the two doses mentioned above. While the primary endpoint in both the studies will be factor VIII (a blood clotting protein) activity level achieved following administration of valoctocogene roxaparvovec, the secondary endpoints will measure annualized factor VIII replacement therapy use rate and annualized bleed rate.
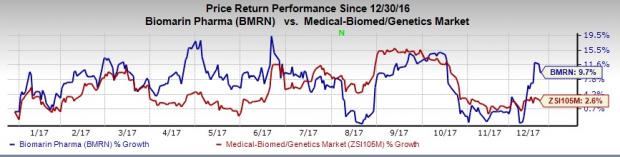
BioMarin’s share price has gained 9.7% in the last 12 months, comparing favorably with the industry’s increase of 2.6%.
We remind investors that earlier this month, BioMarin presented an updated data from a phase I/II study on valoctocogene roxaparvovec at the American Society of Hemophilia. The findings suggested that a one-time infusion of valoctocogene roxaparvovec has potential to eliminate non-stop bleeding for patients suffering severe hemophilia A with a very acceptable safety profile.
The company is also planning to start another phase I/II trial to evaluate valoctocogene roxaparvovec with the 6e13kg/vg dose on approximately 10 patients, who are adeno-associated virus type 5 (AAV5) positive, in the first half of 2018.
Notably, last March, BioMarin had announced that the FDA granted an Orphan Drug designation to valoctocogene roxaparvovec for treatment of patients with hemophilia A.
Additionally, in February, valoctocogene roxaparvovec has been granted an access to Priority Medicines, a regulatory initiative by the European Medicines Agency. This proposal offers an early and enhanced support to optimize regulatory applications. This would help patients get fast benefits from the therapies that promise to improve their quality of life.
It is important to note that the current marketed therapies for hemophilia include Bayer AG’s (OTC:BAYRY) Kovaltry, approved in both the United States and the EU in early 2016 both for children and the adults.
Another company, Alnylam Pharmaceuticals (NASDAQ:ALNY) , is developing its hemophilia candidate, fitusiran, in partnership with Sanofi (NYSE:SNY) . Several mid to late stage studies are also underway on the candidate.
Zacks Rank
Biomarin carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Medical Stocks to Buy Now
Zacks names 5 companies poised to ride a medical breakthrough that is targeting cures for leukemia, AIDS, muscular dystrophy, hemophilia, and other conditions.
New products in this field are already generating substantial revenue and even more wondrous treatments are in the pipeline. Early investors could realize exceptional profits.
Click here to see the 5 stocks >>
Sanofi (SNY): Free Stock Analysis Report
Bayer AG (DE:BAYGN) (BAYRY): Free Stock Analysis Report
BioMarin Pharmaceutical Inc. (BMRN): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.