
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

AVEO Pharmaceuticals Q3 Loss Narrows, Focus On Fotivda

AVEO Pharmaceuticals, Inc. (NASDAQ:AVEO) reported a third-quarter 2017 adjusted loss of 2 cents per share (excluding loss due to change in fair value of warrant liability), narrower than the Zacks Consensus Estimate of 5 cents as well as the year-ago loss of 8 cents. However, including loss related to change in fair value of warrant liability, net loss per share for the quarter was 22 cents.
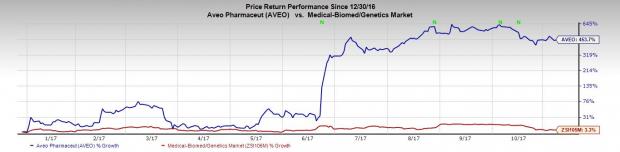
Shares of the company rose more than 1.7% on Tuesday. In fact, AVEO’s share price movement shows that the stock has significantly outperformed the industry on a year-to-date basis. AVEO has surged 453.7% during this period, compared with the industry’s gain of 3.3%.
AVEO’s first drug, Fotivda (tivozanib) received EU approval in August for the first-line treatment of advanced renal cell carcinoma (RCC). The company will receive double-digit royalty payments from EUSA Pharma on net sales of the drug in Europe.
However, Fotivda did not record any sales this quarter and AVEO’s top line mainly comprises collaboration revenues, milestone and other payments. Total collaboration revenues in the third quarter were approximately $4.6 million compared with $1 million in the year-ago quarter. Revenues missed the Zacks Consensus Estimate of $6.5 million.
Quarterly Highlights
Research & development expenses were up 5% to about $4.7 million. However, general and administrative expenses decreased 1.9% year over year to $2.1 million.
Pipeline Updates
In June, the company announced the completion of enrollment in a phase III study, TIVO-3, evaluating Fotivda. Top-line data is expected in the first quarter of 2018. Subsequent to the quarter in October, based on a pre-planned futility analysis, AVEO announced that the study comparing tivozanib with Bayer’s (OTC:BAYRY) Nexavar in patients with refractory advanced RCC will continue unmodified.
The data from this study along with previously completed TIVO-1 study for first-line setting will support the regulatory application for approval of tivozanib in the United States as a first- and third-line treatment for RCC. A potential approval in the United States in this indication will provide further boost to the company.
In September, AVEO announced that EUSA Pharma has opted into a phase I/II TiNino study evaluating Fotivda in combination with Bristol-Myers Squibb Company’s (NYSE:BMY) Opdivo in RCC. EUSA Pharma may use the study data for regulatory or commercial purposes in exchange for a payment of $2 million.
2017 Guidance
AVEO expects that its present cash resources of $37.4 million will allow the company to fund its planned operations through the fourth quarter of 2018.
Our Take
The approval of Fotivda in the EU wass a huge boost for the company as the first-line RCC is a lucrative market in Europe. Moreover, it will also remove AVEO’s dependence on collaboration revenues and milestone payments.
Zacks Rank & Stock to Consider
AVEO Pharma carries a Zacks Rank #3 (Hold). A better-ranked stock in the health care sector is Exelixis, Inc. (NASDAQ:EXEL) ,carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Exelixis’ earnings per share estimates increased from 26 cents to 43 cents for 2017 and from 63 cents to 70 cents for 2018 over the last 30 days. The company delivered positive earnings surprise in all the four trailing quarters with an average beat of 572.92%. The company’s shares are up 78.3% so far this year.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Bayer AG (DE:BAYGN) (BAYRY): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
AVEO Pharmaceuticals, Inc. (AVEO): Free Stock Analysis Report
Original post
Related Articles

Through many years of frustration among gold bugs due to the failure of gold stock prices to leverage the gold prices in a positive way, there were very clear reasons for that...

I know there is the smell of fear in the air when I see my readership double as we reach a point where weekly chart factors come into play. Up until last week, markets have...

Professional traders get paid because of one skill and one skill only: the ability to foresee what the world (or the economy, at least) might look like in six to nine months....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.