
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

AstraZeneca's Lokelma Gets Approval In Japan For Hyperkalaemia

AstraZeneca PLC (NYSE:AZN) announced that the Japanese regulatory authority has granted marketing approval to Lokelma (sodium zirconium cyclosilicate) for the treatment of patients with hyperkalaemia in Japan. Hyperkalemia is a serious condition characterized by high levels of potassium in the blood and Lokelma is a highly selective, oral potassium-removing agent.
Following the nod, Lokelma becomes the first innovative non-resin potassium binder to be approved in Japan.
Lokelma is already marketed across the United States, Canada, Hong Kong, China, Russia and in the EU for the treatment of hyperkalaemia.
Per the company, the nod by Japan’s Ministry of Health, Labour and Welfare (MHLW), was based on positive data from stand-alone studies in Japan as well as global evaluations. It was also supported by the global DIALIZE study, evaluating end-stage renal disease patients on haemodialysis. The same demonstrated the positive efficacy and safety of Lokelma in addressing hyperkalaemia. Lokelma also significantly improved control of pre-dialysis hyperkalaemia compared with placebo in the study.
Patients in Japan can now benefit from Lokelma’s rapid, sustained potassium control and tolerability.
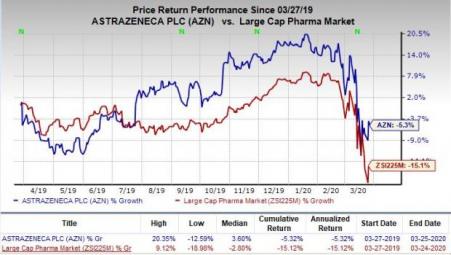
Shares of AstraZeneca have lost 5.3% in the past year compared with the industry’s decrease of 15.1%.
In a separate press release, AstraZeneca announced that it has collaboration with London-based biotech Silence Therapeutics to discover, develop and commercialize small interfering RNA (siRNA) therapeutics for the treatment of cardiovascular, renal, metabolic and respiratory diseases.
The alliance will leverage Silence’s established siRNA platform to identify and progress liver-based targets for addressing such diseases.
Notably, AstraZeneca, which operates in multiple countries across the globe, is focusing on the emerging markets, which represent its largest market in terms of product sales and accounted for 35% of the total total product revenues in 2019.
The company inked a number of deals (Almirall’s respiratory franchise, Bristol-Myers’ diabetes portfolio, Pearl Therapeutics and Omthera Pharmaceuticals) and struck agreements with heavyweight companies like FibroGen, Inc. (NASDAQ:FGEN) .
Zacks Rank & Stocks to Consider
AstraZeneca currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the large cap pharma sector include Pfizer Inc. (NYSE:PFE) and AbbVie Inc. (NYSE:ABBV) , both carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Pfizer’s earnings estimates have been revised 4.9% upward for 2020 over the past 60 days.
AbbVie’s earnings estimates have moved 2% north for 2020 over the past 60 days.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +24.5% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
AstraZeneca PLC (AZN): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
FibroGen, Inc (FGEN): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.