
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

AstraZeneca, Merck's Lynparza sNDA Gets FDA Priority Tag

AstraZeneca (NASDAQ:AZN) AZN and partner Merck MRK announced that the FDA has accepted their supplemental new drug application (sNDA) seeking expanded use of PARP inhibitor Lynparza in breast cancer.
The sNDA is seeking approval of Lynparza as an adjuvant treatment of patients with BRCA-mutated (BRCAm) HER2-negative high-risk early breast cancer previously treated with chemotherapy either before or after surgery.
With the FDA granting priority review to AstraZeneca and Merck’s sNDA, a decision is expected in the first quarter of 2022
The sNDA for the expanded indication was based on data from the OlympiA phase III study. In the study, treatment with Lynparza reduced the risk of invasive breast cancer recurrence, second cancers or death by 42% versus placebo, thereby demonstrating statistically significant and clinically meaningful improvement in invasive disease-free survival (iDFS).
Lynparza is already approved for the treatment of germline BRCAm, HER2-negative, metastatic breast cancer previously treated with chemotherapy based on results from the OlympiAD Phase III trial.
An estimated 2.3 million patients were diagnosed with breast cancer in 2020. Around 91% of all breast cancer patients are diagnosed at an early stage of the disease. If Lynparza is approved for the early breast cancer indication, it can cater to a significantly expanded patient population.
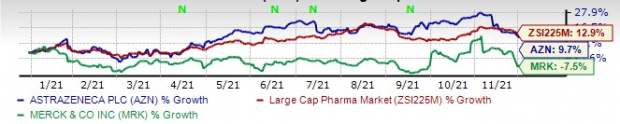
This year so far, AstraZeneca’s shares have risen 9.7% while Merck’s shares have lost 7.5%. The industry has witnessed an increase of 12.9% in the said time frame.
At present, Lynparza, is approved for four cancer types, ovarian, breast, prostate and pancreatic for various patient populations. It has been used to treat over 40,000 patients worldwide. Lynparza is also being evaluated in an earlier-line setting for the approved cancer indications as well as some other cancer types.
Lynparza is being jointly developed and commercialized by AstraZeneca and Merck. The drug generated product sales of $1.72 for AstraZeneca in the first nine months of 2021, and alliance revenues of $721 million for Merck.
Other PARP inhibitors available in the market include Glaxo’s GSK Zejula and Clovis Oncology (NASDAQ:CLVS)’s CLVS Rubraca and Pfizer’s Talzenna.
While Glaxo’s Zejula is approved only for an ovarian cancer indication, Clovis’ Rubraca is approved for BRCA mutated ovarian cancer and metastatic castrate-resistant prostate cancer indications.
A broad development program on Rubraca is currently underway across a variety of solid tumors. Clovis is looking to expand Rubraca’s label into additional cancer types like breast and gastroesophageal cancers, among others.
On the other hand, Glaxo’s Zejula is being evaluated for additional ovarian cancer stages as well as for non-small cell lung cancer and breast cancer
Both Merck and AstraZeneca have a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks Names "Single Best Pick to Double"
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
You know this company from its past glory days, but few would expect that it's poised for a monster turnaround. Fresh from a successful repositioning and flush with A-list celeb endorsements, it could rival or surpass other recent Zacks' Stocks Set to Double like Boston Beer (NYSE:SAM) Company which shot up +143.0% in a little more than 9 months and Nvidia (NASDAQ:NVDA) which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN): Free Stock Analysis Report
GlaxoSmithKline plc (NYSE:GSK): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
Related Articles

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...

Palantir Technologies (NASDAQ:PLTR) continues to sell off. On March 6, PLTR stock fell over 10% on nearly double the daily volume, bringing its 30-day decline to over 27%. A drop...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.