
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Aprea (APRE) Reports FDA Removal of Clinical Hold on Study

Small biotech Aprea Therapeutics (NASDAQ:APRE), Inc. APRE announced that the FDA has removed the full clinical hold on a study evaluating its lead product candidate, eprenetapopt (APR-246), in combination with other cancer drugs for treating lymphoid malignancies.
The study is investigating eprenetapopt in combination with AstraZeneca (NASDAQ:AZN)’s AZN Calquence (acalabrutinib), or with AbbVie ABBV/Roche’s Venclexta (venetoclax) and Roche’s RHHBY (OTC:RHHBY) Rituxan (rituximab) for the given indication.
Aprea Therapeutics addressed the FDA’s concern and received clearance from the regulatory body to resume clinical evaluation of eprenetapopt in non-Hodgkin’s lymphomas.
Venclexta has been developed by AbbVie and Roche. The drug is being jointly commercialized by AbbVie and Roche in the United States while AbbVie markets the drug in ex-U.S. markets under the brand name Venclyxto.
AstraZeneca’s Calquence is approved for chronic lymphocytic leukemia (in frontline as well as relapsed/recurrent disease setting) and previously treated mantle cell lymphoma.
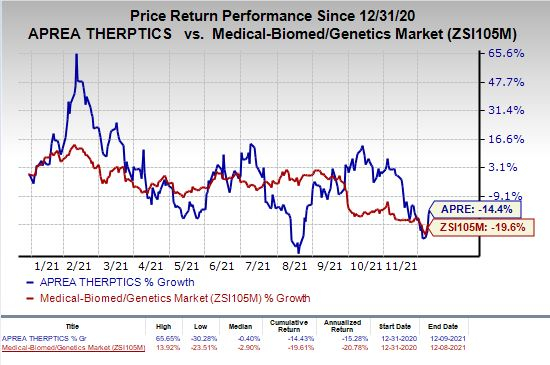
Shares of Aprea Therapeutics were up 2.2% on Thursday following the FDA’s removal of clinical hold on the above-mentioned study on eprenetapopt. The stock has plunged 14.4% so far this year compared with the industry’s decrease of 19.6%.
Aprea Therapeutics is developing eprenetapopt for treating hematologic malignancies and solid tumors.
Please note that in August 2021, the FDA placed a clinical hold on the lymphoid malignancy program owing to concerns over the safety and efficacy data from the phase III frontline study in myelodysplastic syndromes (“MDS”).
The same month, the FDA placed a partial clinical hold on several studies evaluating eprenetapopt in combination with Vidaza (azacitidine), including a phase III frontline MDS. All these studies were put on clinical hold due to concerns about the safety and efficacy data from the frontline MDS study.
The FDA has granted Orphan Drug and Fast Track designations to eprenetapopt for treating MDS as well as acute myeloid leukemia (“AML”). The European Commission has also granted Orphan Drug designation to eprenetapopt for MDS and AML.
Zacks Rank
Aprea Therapeutics currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Stocks Set to Double
Each was handpicked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2021. Previous recommendations have soared +143.0%, +175.9%, +498.3% and +673.0%.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN): Free Stock Analysis Report
Roche Holding AG (OTC:RHHVF) (RHHBY): Free Stock Analysis Report
AbbVie Inc. (NYSE:ABBV): Free Stock Analysis Report
Aprea Therapeutics, Inc. (APRE): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
Related Articles

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...

Palantir Technologies (NASDAQ:PLTR) continues to sell off. On March 6, PLTR stock fell over 10% on nearly double the daily volume, bringing its 30-day decline to over 27%. A drop...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.