
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Alnylam's Givlaari Gets Approval In Europe For Rare Disease

Alnylam Pharmaceuticals Inc. (NASDAQ:ALNY) announced that the European Commission has approved Givlaari (givosiran) for the treatment of acute hepatic porphyria (AHP) in patients aged 12 years or older. The drug will be available as subcutaneous injection. The approval in Europe was expected as the drug had received positive recommendation from the Committee for Medicinal Products for Human Use of the European Medicines Agency in January 2020.
The ultra-rare AHP disease can lead to severe abdominal pain, vomiting and seizures, which can be life-threatening due to the possibility of paralysis and respiratory arrest during attacks.
The approval was based on impressive data from the phase III ENVISION study, which showed that treatment with Givlaari resulted in 74% reduction in the rate of porphyria attacks. The drug is the first and only therapy approved in Europe, which demonstrated prevention of AHP attacks, reduction in chronic pain and improving quality of life.
Please note that Givlaari was approved by the FDA in November last year for the same indication. It has already been launched in the United States and generated $0.2 million in the fourth quarter of 2019. The drug is priced at $575,000 per annum in the U.S. market.
Shares of the company have rallied 31.1% year to date against the industry’s decrease of 7.7%.
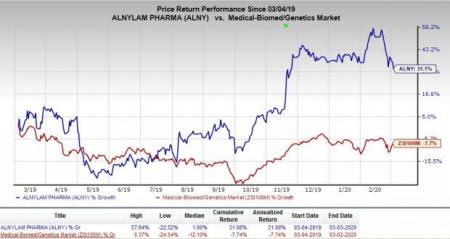
Alnylam is focused on development of novel RNAi-based therapeutics targeting primarily rare-disease indications. The company has several collaborations with leading pharmaceutical and life sciences companies including Novartis (NYSE:NVS) , Roche (OTC:RHHBY) and Ionis Pharmaceuticals (NASDAQ:IONS) among others. It has received approval for two RNAi-based therapeutics — Givlaari and Onpattro — in the United States and Europe in the last 18 months.
Onpattro was approved in 2018 for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The drug generated $166 million in 2019. Apart from its approved drugs, Alnylam is developing several RNAi-based candidates. The company has initiated new drug application (“NDA”) rolling submission for lumasiran, with remaining sections expected to be submitted to the FDA in early 2020. The NDA is seeking approval for lumasiran as a treatment for primary hyperoxaluria type I, a rare-disorder affecting kidneys.
Further, the company expects to advance inclisiran, being evaluated to treat hypercholesterolemia, along with partner Novartis and filed an NDA in December 2019. It is also developing another candidate, fitusiran, in collaboration with Sanofi (PA:SASY) in late-stage study as a treatment for hemophilia A or B.
Zacks Rank
Alnylam currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2019, while the S&P 500 gained and impressive +53.6%, five of our strategies returned +65.8%, +97.1%, +118.0%, +175.7% and even +186.7%.
This outperformance has not just been a recent phenomenon. From 2000 – 2019, while the S&P averaged +6.0% per year, our top strategies averaged up to +54.7% per year.
See their latest picks free >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS): Free Stock Analysis Report
Original post
Related Articles

• Trump’s trade war, inflation data, and last batch of earnings will be in focus this week. • DoorDash’s imminent inclusion in the S&P 500 is likely to trigger a wave of...

The big US stocks dominating markets and investors’ portfolios just finished another earnings season. They reported spectacular collective results including record sales, profits,...

“Quality” stocks with strong fundamentals tend to be rewarding places to stash hard-earned money. Since 2009, investing in a basket of quality stocks over a standard index has...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review



Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.