
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Alnylam Starts Rolling NDA Submission For RNAi Candidate

Alnylam Pharmaceuticals, Inc. (NASDAQ:ALNY) announced that it has initiated submission of a rolling New Drug Application (NDA) to the FDA for lead candidate patisiran. The candidate is an investigational RNAi therapeutic targeting transthyretin (TTR) for the treatment of hereditary ATTR (hATTR) amyloidosis. The rolling submission allows completed portions of an NDA to be reviewed by the FDA on an ongoing basis.
Alnylam has also requested priority review of application for patisiran, if granted, it might result in a six-month review process.
Notably, the NDA filing is based on promising results from the APOLLO phase III study that met its primary as well as all secondary endpoints. The patients who were administered patisiran experienced significant improvement in quality of life compared to placebo. In fact, APOLLO was the largest randomized study ever completed in this disease. The study also demonstrated the first-ever positive results for an RNAi therapeutic.
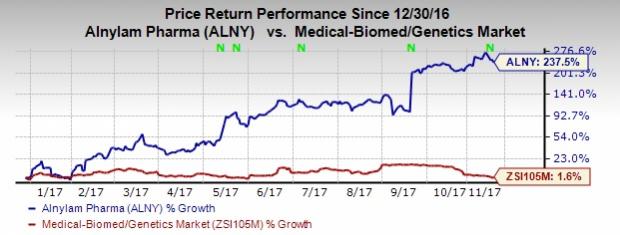
So far this year, shares of Alnylam have skyrocketed 237.5% compared with the industry’s gain of 1.6%.
Recently, Alnylam also announced that the Committee for Medicinal Products for Human Use (“CHMP”) of the European Medicines Agency (“EMA”) has granted an accelerated assessment for patisiran. With the accelerated assessment, the company can expect the review timeline to be reduced from the typical 210 days to 150 once the marketing authorization application (MAA) is filed and validated in the EU.
Alnylam and its partner Sanofi (NYSE:SNY) plan to submit Marketing Authorisation Application to the European Medicines Agency around the year-end.
Meanwhile, Sanofi is preparing for regulatory filings for patisiran in Japan, Brazil and other countries , beginning in the first half of 2018. Once it gets the required regulatory approvals, Alnylam will commercialize patisiran in the United States, Canada and Western Europe with Sanofi commercializing the product in the rest of the world.
Going forward, the potential approval of patisiran is likely to be a huge boost for the people suffering from this often fatal disease.
Zacks Rank & Stocks to Consider
Alnylam carries a Zacks Rank #3 (Hold). Some better-ranked health care stocks in the same space include Ligand Pharmaceuticals Incorporated (NASDAQ:LGND) and Corcept Therapeutics Incorporated (NASDAQ:CORT) holding a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Ligand’s earnings per share estimates have moved up $3.68 to $3.70 for 2018 over the last 60 days. The company pulled off positive earnings surprises in two of the trailing four quarters, with an average beat of 8.22%. The share price of the company has increased 39.4% year to date.
Corcept’searnings per share estimates have moved up from 45 cents to 47 cents for 2017 and from 77 cents to 88 cents for 2018 over the last 60 days. The company delivered positive earnings surprises in two of the trailing four quarters, with an average beat of 14.32%. The share price of the company has increased 162.4% year to date.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Sanofi (SNY): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND): Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

As the digital economy starts to go online across businesses and the world, investors have to be aware of the companies and services that will be at the forefront of this...

Wall Street Indexes remain under pressure today but have held above the lows we saw on Tuesday as the Trump administration tariffs came into force. The announcement of tariffs on...

These stocks provide a compelling case as safe-haven stocks in the face of an escalating trade war. Each company operates within sectors that are relatively resilient to economic...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.