
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Allergan's Vraylar Positive For Bipolar Disease In Phase III

Allergan plc (NYSE:AGN) , along with partner Gedeon Richter, announced positive top-line results from a second pivotal phase III study on its schizophrenia drug Vraylar.
The study evaluated Vraylar (cariprazine) for treatment of adult patients with major depressive episodes associated with bipolar I disorder (bipolar I depression).
Notably, Vraylar is already approved in the United States and the EU for the acute treatment of manic or mixed episodes associated with bipolar I disorder as well as for schizophrenia. Additionally, the FDA approved the drug last month for an expanded use as a maintenance therapy for treatment of schizophrenia.
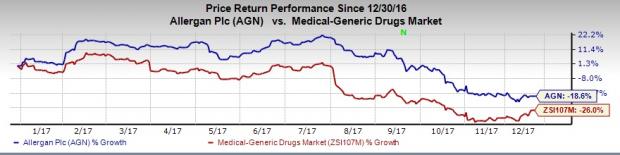
Shares of Allergan have declined 18.6% this year so far, comparing favorably with the industry’s decrease of 26%.
The phase III (RGH-MD-54) study was investigating two doses of Vraylar (1.5 mg and 3 mg) in a total of 488 patients with bipolar I depression. The study met its primary endpoints and showed a significant improvement in patients compared with placebo. The drug was generally well-tolerated in the study. However, we also note that of the total number of patients, around 5.0% being treated with Vraylar discontinued the trial due to adverse events in comparison to 2.5% treated with placebo.
Based on the above reported data, the company is planning to submit regulatory applications to the FDA in the second half of 2018.
Significantly, Allergan has a licensing agreement with Gideon Richter, a Hungarian multinational pharmaceutical and biotechnology company, to co-develop and market Vraylar in the United States as well as Canada.
Vraylar has performed well in the first nine months of 2017, recording sales of $200.1 million compared with $51.1 million, registered in the same period a year ago. The drug is likely to be a key driver of Allergan’s top line in 2018.
Per National Alliance of Mental Illness, bipolar disorder is one of the most common mental diseases and the third most common cause of hospitalization in the United States for both youth and adults aged between 18 and 44 years. Moreover, 2.6% of adults in the country live with bipolar disorder. Hence, approval of the drug for the expanded indication will provide the company with access to a wider patient population base with bipolar issues.
Other players in the bipolar disorder and schizophrenia treatment market include AstraZeneca plc's (NYSE:AZN) Seroquel XR, Johnson & Johnson's (NYSE:JNJ) Risperdal Consta and Alkermes plc's (NASDAQ:ALKS) Aristada.
Zacks Rank
Allergan carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks Editor-in-Chief Goes "All In" on This Stock
Full disclosure, Kevin Matras now has more of his own money in one particular stock than in any other. He believes in its short-term profit potential and also in its prospects to more than double by 2019. Today he reveals and explains his surprising move in a new Special Report.
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Allergan PLC. (AGN): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Alkermes PLC (ALKS): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

At the end of February, Lululemon (NASDAQ:LULU), DoorDash (NASDAQ:DASH), and Ulta Beauty (NASDAQ:ULTA) were among the Most Upgraded Stocks tracked by MarketBeat. Investors should...

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.