
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Allergan/Paratek's Acne Candidate NDA Filing Accepted By FDA

Allergan plc (NYSE:AGN) along with partner Paratek Pharmaceuticals (NASDAQ:PRTK) announced that its new drug application (NDA) for its investigational, once-daily, Seysara (sarecycline) has been accepted for review by the FDA for treatment of patients aged nine years and above with moderate to severe acne vulgaris. The company expects a response from the FDA in the second half of 2018.
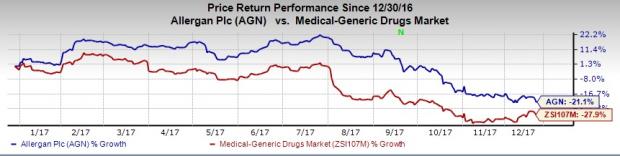
A look at Allergan’s share price movement so far this year shows that the stock has outperformed the industry. Though the stock has lost 21.1%, the industry has decreased 27.9%. Also shares of Paratek were up 15.2% during the period.
Allergan completed its NDA submission for Seysara in October, based on positive data from two phase III (n=2002) studies — SC1401 and SC1402 — respectively. The trials met the primary endpoints and revealed that patients treated with Seysara (1.5 mg/kg) demonstrated a statistically significant improvement in acne severity compared with placebo at week 12.
Notably, Allergan owns the rights for development and commercialization of Seysara in the United States while Paratek retains the rights in the ex-U.S. markets.
Among other players conducting trials on candidates in the same space, Foamix Pharmaceuticals Ltd. (NASDAQ:FOMX) is developing FMX101 in late-stage studies for treating moderate to severe acne.
We remind investors that Allergan has more than 55 projects in mid-to-late stage development. The company has extended its R&D pipeline to adjacent categories like NASH, Parkinson's disease and gene therapy with many promising phase II/III programs on development track.
Allergan also boasts a strong branded pipeline with meaningful data read-outs expected next year.
Zacks Rank & Key Pick
Allergan carries a Zacks Rank #3 (Hold). A better-ranked stock in the health care sector is Corcept Therapeutics Incorporated (NASDAQ:CORT) carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Corcept’s earnings per share estimates have moved up from 78 cents to 88 cents for 2018 over the last 60 days. The company delivered positive earnings surprises in two of the trailing four quarters with an average beat of 14.32%. Share price of the company has skyrocketed 117.7% year to date.
Zacks’ Best Private Investment Ideas
While we are happy to share many articles like this on the website, our best recommendations and most in-depth research are not available to the public.
Starting today, for the next month, you can follow all Zacks' private buys and sells in real time. Our experts cover all kinds of trades… from value to momentum . . . from stocks under $10 to ETF and option moves . . . from stocks that corporate insiders are buying up to companies that are about to report positive earnings surprises. You can even look inside exclusive portfolios that are normally closed to new investors.
Click here for Zacks' private trades >>
Allergan PLC. (AGN): Free Stock Analysis Report
Paratek Pharmaceuticals, Inc. (PRTK): Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT): Free Stock Analysis Report
Foamix Pharmaceuticals Ltd. (FOMX): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

The markets have been sluggish this week as investors hope for a jolt later in the week when AI juggernaut NVIDIA Corporation (NASDAQ:NVDA) reports fourth quarter and year-end...

On Friday, a wave of selling pressure swept across the US equity markets, leaving a trail of losses. The S&P 500 closed down 1.7%, the DOW slid 1.69%, and the NASDAQ tumbled a...

Palantir remains highly valued with a 460x P/E ratio and a 42.5x P/B ratio, far above its peers. The stock's beta of 2.81 signals high volatility, meaning sharp moves in both...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review




Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.