
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Acorda Resubmits NDA For Parkinson's Disease Candidate Inbrija

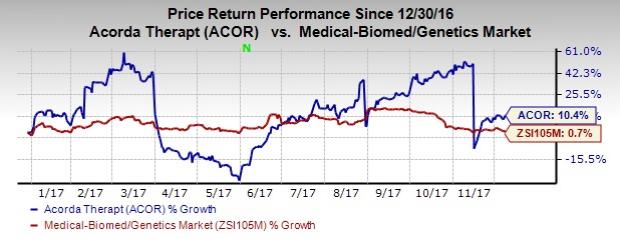
Acorda Therapeutics (NASDAQ:ACOR) announced that it has resubmitted the new drug application (NDA) for its late stage pipeline candidate, Inbrija, to the FDA. Following the news, shares of the company gained by about 1.9%. So far this year, shares of the company have increased 10.4% compared with the industry’s gain of 0.7%.
Currently, the company is seeking approval for Inbrija in the United States as a treatment option for symptoms of OFF periods in people with Parkinson’s taking a carbidopa / levodopa regimen.
We remind investors that in August the company received a refusal to file (RTF) letter from the FDA in connection with the new drug application (NDA) that it first submitted in June. However, the FDA declared the application to be incomplete after a preliminary review. As a result, the regulatory body required additional supporting information to review the application.
Notably, the FDA stated two main reasons for the RTF — date specification as to when the manufacturing site can be ready for inspection and questions related to drug master production record. Additionally, the FDA asked for some extra data, unrelated to the main issues of the RTF. Acorda claims that it has addressed these two issues in its resubmission.
Acorda had submitted the NDA as a 505(b)(2) application. The FDA is expected to inform the company within 74 days if the submission is complete and requires a full review.
In this context, we remind investors that in November, Acorda decided to immediately discontinue the phase III study on one of its lead Parkinson’s disease candidates, tozadenant. The decision was based on some serious safety issues observed in the study.
Apart from Acorda, many companies are trying to introduce treatments for Parkinson’s disease in the market namely, Prothena Corporation’s (NASDAQ:PRTA) PRX002, AstraZeneca’s (NYSE:AZN) MEDI1341, Prexton Therapeutics’ Foliglurax and Prana Biotechnology’s PBT434.
Also, Adamas Pharmaceuticals’ (NASDAQ:ADMS) Gocovri received an FDA approval in August for treating dyskinesia in patients with Parkinson’s disease.
Zacks Rank
Acorda carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Acorda Therapeutics, Inc. (ACOR): Free Stock Analysis Report
Prothena Corporation PLC (PRTA): Free Stock Analysis Report
Adamas Pharmaceuticals, Inc. (ADMS): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Regimes are changing in the market, and this could mean a few things, but today, it means that volatility is back. Whenever these shifts come, specifically to the S&P 500...

There are more than two reasons why NVIDIA’s (NASDAQ:NVDA) stock price can rally another 30% or more in 2025, but the two that underpin the others are data center and automotive...

Stocks fell sharply, with the S&P 500 leading the decline, finishing the day down almost 1.6% at 5,860. Meanwhile, the Nasdaq 100 dropped nearly 2.75%, closing at 20,550. This...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.